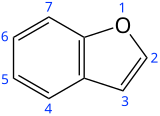
| |||
| |||
| Names | |||
|---|---|---|---|
| Preferred IUPAC name
1-Benzofuran[1] | |||
| Other names | |||
| Identifiers | |||
3D model (JSmol)
|
|||
| 107704 | |||
| ChEBI | |||
| ChEMBL | |||
| ChemSpider | |||
| DrugBank | |||
| ECHA InfoCard | 100.005.439 | ||
| EC Number |
| ||
| 260881 | |||
| KEGG | |||
PubChem CID
|
|||
| RTECS number |
| ||
| UNII | |||
| UN number | 1993 | ||
CompTox Dashboard (EPA)
|
|||
| |||
| |||
| Properties | |||
| C8H6O | |||
| Molar mass | 118.135 g·mol−1 | ||
| Melting point | −18 °C (0 °F; 255 K) | ||
| Boiling point | 173 °C (343 °F; 446 K) | ||
| Hazards | |||
| GHS labelling: | |||
 
| |||
| Warning | |||
| H226, H351, H412 | |||
| P201, P202, P210, P233, P240, P241, P242, P243, P273, P280, P281, P303+P361+P353, P308+P313, P370+P378, P403+P235, P405, P501 | |||
| Lethal dose or concentration (LD, LC): | |||
LD50 (median dose)
|
500 mg/kg (mice).[2] | ||
| Related compounds | |||
Related compounds
|
Benzothiophene, Indole, Indene, 2-Cumaranone | ||
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa).
| |||
Benzofuran is the heterocyclic compound consisting of fused benzene and furan rings. This colourless liquid is a component of coal tar. Benzofuran is the structural nucleus (parent compound) of many related compounds with more complex structures. For example, psoralen is a benzofuran derivative that occurs in several plants.
Production[edit]
Benzofuran is extracted from coal tar. It is also obtained by dehydrogenation of 2-ethylphenol.[2]
Laboratory methods[edit]
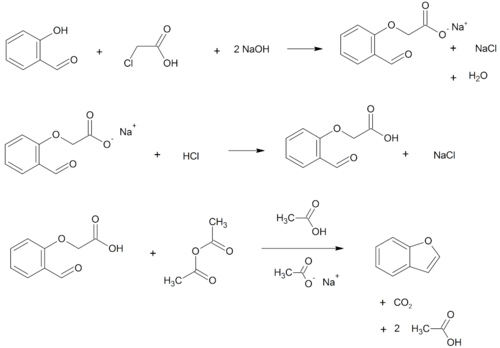
Benzofurans can be prepared by various methods in the laboratory. Notable examples include:
- O-alkylation of salicylaldehyde with chloroacetic acid followed by dehydration (cyclication) of the resulting ether and decarboxylation.[3]
- Diels–Alder reaction[clarification needed] of nitro vinyl furans with various dienophiles:[7]
Related compounds[edit]
- Substituted benzofurans
- Dibenzofuran, an analog with a second fused benzene ring.
- Furan, an analog without the fused benzene ring.
- Indole, an analog with a nitrogen instead of the oxygen atom.
- Benzothiophene, an analog with a sulfur instead of the oxygen atom.
- Isobenzofuran, the isomer with oxygen in the adjacent position.
- Aurone
- Thunberginol F
References[edit]
- ^ a b "Front Matter". Nomenclature of Organic Chemistry : IUPAC Recommendations and Preferred Names 2013 (Blue Book). Cambridge: The Royal Society of Chemistry. 2014. p. 218. doi:10.1039/9781849733069-FP001. ISBN 978-0-85404-182-4.
- ^ a b Collin, G.; Höke, H. (2007). "Benzofurans". Ullmann's Encyclopedia of Industrial Chemistry. Weinheim: Wiley-VCH. doi:10.1002/14356007.l03_l01. ISBN 978-3527306732.
- ^ Burgstahler, A. W.; Worden, L. R. (1966). "Coumarone" (PDF). Organic Syntheses. 46: 28; Collected Volumes, vol. 5, p. 251.
- ^ Perkin, W. H. (1870). "XXIX. On some New Bromine Derivatives of Coumarin". Journal of the Chemical Society. 23: 368–371. doi:10.1039/JS8702300368.
- ^ Perkin, W. H. (1871). "IV. On some New Derivatives of Coumarin". Journal of the Chemical Society. 24: 37–55. doi:10.1039/JS8712400037.
- ^ Bowden, K.; Battah, S. (1998). "Reactions of Carbonyl Compounds in Basic Solutions. Part 32. The Perkin Rearrangement". Journal of the Chemical Society, Perkin Transactions 2. 1998 (7): 1603–1606. doi:10.1039/a801538d.
- ^ Kusurkar, R. S.; Bhosale, D. K. (1990). "Novel Synthesis of Benzosubstituted Benzofurans Via Diels-Alder Reaction". Synthetic Communications. 20 (1): 101–109. doi:10.1080/00397919008054620.
- ^ Fürstner, Alois & Davies, Paul (2005). "Heterocycles by PtCl2-Catalyzed Intramolecular Carboalkoxylation or Carboamination of Alkynes". Journal of the American Chemical Society. 127 (43): 15024–15025. doi:10.1021/ja055659p. hdl:11858/00-001M-0000-0025-AA5A-1. PMID 16248631.




Well, that’s interesting to know that Psilotum nudum are known as whisk ferns. Psilotum nudum is the commoner species of the two. While the P. flaccidum is a rare species and is found in the tropical islands. Both the species are usually epiphytic in habit and grow upon tree ferns. These species may also be terrestrial and grow in humus or in the crevices of the rocks.
View the detailed Guide of Psilotum nudum: Detailed Study Of Psilotum Nudum (Whisk Fern), Classification, Anatomy, Reproduction