| |
| Names | |
|---|---|
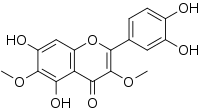
| Preferred IUPAC name
2-(3,4-Dihydroxyphenyl)-5,7-dihydroxy-3,6-dimethoxy-4H-1-benzopyran-4-one | |
Other names
| |
| Identifiers | |
3D model (JSmol)
|
|
| ChEBI | |
| ChEMBL | |
| ChemSpider | |
PubChem CID
|
|
| UNII | |
CompTox Dashboard (EPA)
|
|
| |
| |
| Properties | |
| C17H14O8 | |
| Molar mass | 346.291 g·mol−1 |
| Density | 1.659 g/mL |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa).
| |
Axillarin is an O-methylated flavonol. It can be found in Pulicaria crispa, Filifolium sibiricum, Inula britannica,[1] Wyethia bolanderi in Balsamorhiza macrophylla[2] and in Tanacetum vulgare.[3] It can also be synthesized.[4]
Glycosides
[edit]Axillarin 7-O-β-D-glucoside can be found in Tagetes mendocina, a plant used in traditional herbal medicine the Andean provinces of Argentina.[5]
References
[edit]- ^ Jung Park, Eun; Kim, Youngleem; Kim, Jinwoong (2000). "Acylated Flavonol Glycosides from the Flower ofInula britannica". Journal of Natural Products. 63 (1): 34–36. doi:10.1021/np990271r. PMID 10650074.
- ^ McCormick, Susan; Robson, Kathleen; Bohm, Bruce (1985). "Methylated flavonols from Wyethia bolanderi and Balsamorhiza macrophylla". Phytochemistry. 24 (9): 2133. doi:10.1016/S0031-9422(00)83143-X.
- ^ Álvarez, Ángel L.; Habtemariam Solomon; Juan-Badaturuge Malindra; Jackson Caroline; Parra Francisco (2011). "In vitro anti HSV-1 and HSV-2 activity of Tanacetum vulgare extracts and isolated compounds: An approach to their mechanisms of action". Phytotherapy Research (Submitted manuscript). 25 (2): 296–301. doi:10.1002/ptr.3382. PMID 21171142. S2CID 9011931.
- ^ Fukui, K.; Nakayama, M.; Horie, T. (1968). "The syntheses of axillarin and its related compounds". Experientia. 24 (8): 769–770. doi:10.1007/BF02144856. S2CID 33789154.
- ^ Guillermo Schmeda-Hirschmann, Alejandro Tapia, Cristina Theoduloz, Jaime Rodrıguez, Susana Lopez and Gabriela Egly Feresin (2004). "Free Radical Scavengers and Antioxidants from Tagetes mendocina" (PDF). Verlag der Zeitschrift für Naturforschung. 59c: 345–353.
{{cite journal}}: CS1 maint: multiple names: authors list (link)
Well, that’s interesting to know that Psilotum nudum are known as whisk ferns. Psilotum nudum is the commoner species of the two. While the P. flaccidum is a rare species and is found in the tropical islands. Both the species are usually epiphytic in habit and grow upon tree ferns. These species may also be terrestrial and grow in humus or in the crevices of the rocks.
View the detailed Guide of Psilotum nudum: Detailed Study Of Psilotum Nudum (Whisk Fern), Classification, Anatomy, Reproduction