 | |
| Clinical data | |
|---|---|
| Routes of administration | Oral |
| ATC code |
|
| Legal status | |
| Legal status |
|
| Pharmacokinetic data | |
| Metabolism | Hepatic |
| Excretion | Renal |
| Identifiers | |
| |
| CAS Number | |
| PubChem CID | |
| DrugBank | |
| ChemSpider | |
| UNII | |
| ChEMBL | |
| CompTox Dashboard (EPA) | |
| Chemical and physical data | |
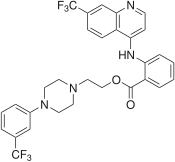
| Formula | C30H26F6N4O2 |
| Molar mass | 588.554 g·mol−1 |
| 3D model (JSmol) | |
| |
| |
| (verify) | |
Antrafenine (Stakane) is a phenylpiperazine derivative drug invented in 1979.[1] It acts as an analgesic and anti-inflammatory drug with similar efficacy to naproxen,[2] but is not widely used as it has largely been replaced by newer drugs.
Synthesis[edit]

Method E: The reaction between 2-[4-[3-(trifluoromethyl)phenyl]-1-piperazinyl]ethanol [40004-29-3] (1) and Isatoic anhydride [118-48-9] (2) goes on to give 4-(3-(Trifluoromethyl)phenyl)piperazine-1-ethyl 2-aminobenzoate [51941-08-3] (3).
Method G: Alkylation with 4-chloro-7-(trifluoromethyl)quinoline [346-55-4] (4) completed the synthesis of antrafenine (5).
See also[edit]
References[edit]
- ^ a b Manoury PM, Dumas AP, Najer H, Branceni D, Prouteau M, Lefevre-Borg FM (May 1979). "Synthesis and analgesic activities of some (4-substituted phenyl-1-piperazinyl)alkyl 2-aminobenzoates and 2-aminonicotinates". Journal of Medicinal Chemistry. 22 (5): 554–9. doi:10.1021/jm00191a017. PMID 458805.
- ^ Leatham PA, Bird HA, Wright V, Seymour D, Gordon A (1983). "A double blind study of antrafenine, naproxen and placebo in osteoarthrosis". European Journal of Rheumatology and Inflammation. 6 (2): 209–11. PMID 6673985.
- ^ Don Pierre Rene Lucien Giudicelli, et al. U.S. patent 4,017,623 (1977 to Synthelabo SA).
- ^ Don Pierre Rene Lucien Giudicelli, et al. U.S. patent 3,935,229 (1976 to Synthelabo SA).
- ^ Don Pierre Rene Lucien Giudicelli, et al. U.S. patent 3,953,449 (1976 to Synthelabo SA).
Well, that’s interesting to know that Psilotum nudum are known as whisk ferns. Psilotum nudum is the commoner species of the two. While the P. flaccidum is a rare species and is found in the tropical islands. Both the species are usually epiphytic in habit and grow upon tree ferns. These species may also be terrestrial and grow in humus or in the crevices of the rocks.
View the detailed Guide of Psilotum nudum: Detailed Study Of Psilotum Nudum (Whisk Fern), Classification, Anatomy, Reproduction