| |||
| |||
| Names | |||
|---|---|---|---|
| Preferred IUPAC name
1,2-Difluoroethene | |||
| Other names
1,2-Difluoroethylene
sym-Difluoroethylene Ethene, 1,2-difluoro-,(Z)- cis-Difluoroethene | |||
| Identifiers | |||
| |||
3D model (JSmol)
|
| ||
| ChemSpider | |||
PubChem CID
|
|||
| |||
| |||
| Properties | |||
| C2H2F2 | |||
| Molar mass | 64.035 g·mol−1 | ||
| Boiling point | -36.0±8.0 °C | ||
| -60.0·10−6 cm3/mol | |||
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa).
| |||
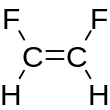
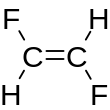
1,2-Difluoroethylene, also known as 1,2-difluoroethene, is an organofluoride with the molecular formula C2H2F2. It can exist as either of two geometric isomers, cis-1,2-difluoroethylene or trans-1,2-difluoroethylene.
It is regarded as a hazardous chemical for being toxic by inhalation, and a volatile chemical, and it causes irritation when it comes into contact with the skin and mucous membranes.
E-Z relative stability
[edit]For most 1,2-disubstituted compounds that exhibit cis–trans isomerism, the trans (E) isomer is more stable than the cis (Z) isomer. However, 1,2-difluoroethylene has the opposite situation, with the cis more stable than the trans by 0.9 kcal/mol.[1]
See also
[edit]References
[edit]- ^ Craig, Norman C.; Entemann, Eric A. (July 1961). "Thermodynamics of cis-trans Isomerizations. The 1,2-Difluoroethylenes". Journal of the American Chemical Society. 83 (14): 3047–3050. doi:10.1021/ja01475a019.



Well, that’s interesting to know that Psilotum nudum are known as whisk ferns. Psilotum nudum is the commoner species of the two. While the P. flaccidum is a rare species and is found in the tropical islands. Both the species are usually epiphytic in habit and grow upon tree ferns. These species may also be terrestrial and grow in humus or in the crevices of the rocks.
View the detailed Guide of Psilotum nudum: Detailed Study Of Psilotum Nudum (Whisk Fern), Classification, Anatomy, Reproduction