| |
| Names | |
|---|---|
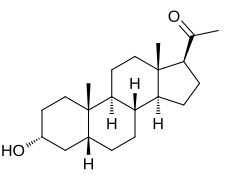
| IUPAC name
3α-Hydroxy-5β-pregnan-20-one
| |
| Systematic IUPAC name
1-[(1S,3aS,3bR,5aR,7R,9aS,9bS,11aS)-7-Hydroxy-9a,11a-dimethylhexadecahydro-1H-cyclopenta[a]phenanthren-1-yl]ethan-1-one | |
| Other names
Eltanolone; 5β-Pregnan-3α-ol-20-one; 3α,5β-Tetrahydroprogesterone; 3α,5β-THP; 3α-Hydroxy-5β-tetrahydroprogesterone
| |
| Identifiers | |
3D model (JSmol)
|
|
| ChEBI | |
| ChEMBL | |
| ChemSpider | |
| ECHA InfoCard | 100.162.192 |
PubChem CID
|
|
| UNII | |
CompTox Dashboard (EPA)
|
|
| |
| |
| Properties | |
| C21H34O2 | |
| Molar mass | 318.501 g·mol−1 |
| Pharmacology | |
| Intravenous injection[1] | |
| Pharmacokinetics: | |
| 0.9–3.5 hours[1][2][3] | |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa).
| |
Pregnanolone, also known as eltanolone, is an endogenous inhibitory neurosteroid which is produced in the body from progesterone.[4] It is closely related to allopregnanolone, which has similar properties.[4]
Biological activity[edit]
Pregnanolone is a positive allosteric modulator of the GABAA receptor,[4] as well as a negative allosteric modulator of the glycine receptor.[5]
Biological function[edit]
Pregnanolone has sedative, anxiolytic, anesthetic, and anticonvulsant effects.[4][5][1] During pregnancy, pregnanolone and allopregnanolone are involved in sedation and anesthesia of the fetus.[6][7]
Biochemistry[edit]
Pregnanolone is synthesized from progesterone via the enzymes 5β-reductase and 3α-hydroxysteroid dehydrogenase, with 5β-dihydroprogesterone occurring as a metabolic intermediate. The elimination half-life of pregnanolone is between 0.9 and 3.5 hours.[1][2][3]
Chemistry[edit]
Pregnanolone, also known as 3α,5β-tetrahydroprogesterone (3α,5β-THP) or as 5β-pregnan-3α-ol-20-one, is a naturally occurring pregnane steroid and a derivative of progesterone. Related compounds include allopregnanolone (3α,5α-THP; brexanolone), epipregnanolone (3β,5β-THP), hydroxydione, isopregnanolone (3β,5α-THP), and renanolone.
History[edit]
Pregnanolone was first isolated from the urine of pregnant women in 1937.[1] Its anesthetic properties were first demonstrated in animals in 1957.[1]
Research[edit]
Pregnanolone was investigated for clinical use as a general anesthetic under the name eltanolone (INN), but produced unwanted side effects such as convulsions on occasion, and for this reason, was never marketed.[5][8][1]
References[edit]
- ^ a b c d e f g Carl P, Høgskilde S, Lang-Jensen T, et al. (October 1994). "Pharmacokinetics and pharmacodynamics of eltanolone (pregnanolone), a new steroid intravenous anaesthetic, in humans". Acta Anaesthesiol Scand. 38 (7): 734–41. doi:10.1111/j.1399-6576.1994.tb03987.x. PMID 7839787. S2CID 22005284.
- ^ a b Gray HS, Holt BL, Whitaker DK, Eadsforth P (March 1992). "Preliminary study of a pregnanolone emulsion (Kabi 2213) for i.v. induction of general anaesthesia". Br J Anaesth. 68 (3): 272–6. doi:10.1093/bja/68.3.272. PMID 1547051. S2CID 19193898.
- ^ a b Carl P, Høgskilde S, Nielsen JW, Sørensen MB, Lindholm M, Karlen B, Bäckstrøm T (March 1990). "Pregnanolone emulsion. A preliminary pharmacokinetic and pharmacodynamic study of a new intravenous anaesthetic agent". Anaesthesia. 45 (3): 189–97. doi:10.1111/j.1365-2044.1990.tb14683.x. PMID 2334030. S2CID 28358731.
- ^ a b c d Reddy DS (2003). "Pharmacology of endogenous neuroactive steroids". Crit Rev Neurobiol. 15 (3–4): 197–234. doi:10.1615/critrevneurobiol.v15.i34.20. PMID 15248811.
- ^ a b c Jürgen Schüttler; Helmut Schwilden (8 January 2008). Modern Anesthetics. Springer Science & Business Media. pp. 278–. ISBN 978-3-540-74806-9.
- ^ Mellor DJ, Diesch TJ, Gunn AJ, Bennet L (2005). "The importance of 'awareness' for understanding fetal pain". Brain Res. Brain Res. Rev. 49 (3): 455–71. doi:10.1016/j.brainresrev.2005.01.006. PMID 16269314. S2CID 9833426.
- ^ Lagercrantz H, Changeux JP (2009). "The emergence of human consciousness: from fetal to neonatal life". Pediatr. Res. 65 (3): 255–60. doi:10.1203/PDR.0b013e3181973b0d. PMID 19092726. S2CID 39391626.
[...] the fetus is sedated by the low oxygen tension of the fetal blood and the neurosteroid anesthetics pregnanolone and the sleep-inducing prostaglandin D2 provided by the placenta (36).
- ^ Norman Calvey; Norton Williams (21 January 2009). Principles and Practice of Pharmacology for Anaesthetists. John Wiley & Sons. pp. 110–. ISBN 978-1-4051-9484-6.
Well, that’s interesting to know that Psilotum nudum are known as whisk ferns. Psilotum nudum is the commoner species of the two. While the P. flaccidum is a rare species and is found in the tropical islands. Both the species are usually epiphytic in habit and grow upon tree ferns. These species may also be terrestrial and grow in humus or in the crevices of the rocks.
View the detailed Guide of Psilotum nudum: Detailed Study Of Psilotum Nudum (Whisk Fern), Classification, Anatomy, Reproduction