Citation bot (talk | contribs) Add: pmc. | You can use this bot yourself. Report bugs here. | Suggested by Headbomb | via #UCB_webform |
AAPS PharmSci |
||
| Line 52: | Line 52: | ||
===Pharmacokinetic/pharmacodynamic modeling=== |
===Pharmacokinetic/pharmacodynamic modeling=== |
||
[[File:Pharmacokinetics.svg|thumb|upright|Basic pharmacokinetic processes affecting the fate of ingested substances. Nonlinear mixed-effects modeling can be used to estimate the population-level effects of these processes while also modeling the individual variation between subjects.]] |
[[File:Pharmacokinetics.svg|thumb|upright|Basic pharmacokinetic processes affecting the fate of ingested substances. Nonlinear mixed-effects modeling can be used to estimate the population-level effects of these processes while also modeling the individual variation between subjects.]] |
||
[[PK/PD models]] for describing [[Dose–response_relationship|exposure-response relationships]] such as the [[Intrinsic_activity|Emax model]] can be formulated as nonlinear mixed-effects models.<ref>{{cite journal |last1=Jonsson |first1=EN |last2=Karlsson |first2=MO |last3= Wade |first3=JR |year=2000 |title=Nonlinearity detection: advantages of nonlinear mixed-effects modeling |journal=AAPS |
[[PK/PD models]] for describing [[Dose–response_relationship|exposure-response relationships]] such as the [[Intrinsic_activity|Emax model]] can be formulated as nonlinear mixed-effects models.<ref>{{cite journal |last1=Jonsson |first1=EN |last2=Karlsson |first2=MO |last3= Wade |first3=JR |year=2000 |title=Nonlinearity detection: advantages of nonlinear mixed-effects modeling |journal=AAPS PharmSci|volume=2 |issue=3 |pages=E32 |doi=10.1208/ps020332 |pmid=11741248 |pmc=2761142 }}</ref> The mixed-model approach allows modeling of both population level and individual differences in effects that have a nonlinear effect on the observed outcomes, for example the rate at which a compound is being metabolized or distributed in the body. |
||
==See also== |
==See also== |
||
Revision as of 19:46, 27 September 2020
| Part of a series on |
| Regression analysis |
|---|
| Models |
| Estimation |
| Background |
Nonlinear mixed-effects models constitue a class of statistical models generalizing linear mixed-effects models. Like linear mixed-effects models, they are particularly useful in settings where there are multiple measurements within the same statistical units or when there are dependencies between measurements on related statistical units. Nonlinear mixed-effects models are applied in many fields including medicine, public health, pharmacology, and ecology.[1][2]
Definition
While any statistical model containing both fixed effects and random effects is an example of a nonlinear mixed-effects model, the most commonly used models are members of the class of nonlinear mixed-effects models for repeated measures[1]
where
- is the number of groups/subjects,
- is the number of observations for the th group/subject,
- is a real-valued differentiable function of a group-specific parameter vector and a covariate vector ,
- is modeled as a linear mixed-effects model where is a vector of fixed effects and is a vector of random effects associated with group , and
- is a random variable describing additive noise.
Estimation
When the model is only nonlinear in fixed effects and the random effects are Gaussian, maximum-likelihood estimation can be done using nonlinear least squares methods, although asymptotic properties of estimators and test statistics may differ from the conventional general linear model. In the more general setting, there exist several methods for doing maximum-likelihood estimation or maximum a posteriori estimation in certain classes of nonlinear mixed-effects models – typically under the assumption of normally distributed random variables. A popular approach is the Lindstrom-Bates algorithm[3] which relies on iteratively optimizing a nonlinear problem, locally linearizing the model around this optimum and then employing conventional methods from linear mixed-effects models to do maximum likelihood estimation. Stochastic approximation of the expectation-maximization algorithm gives an alternative approach for doing maximum-likelihood estimation.[4]
Applications
Disease progression modeling
Nonlinear mixed-effects models have been used for modeling progression of disease.[5] In progressive disease, the temporal patterns of progression on outcome variables may follow a nonlinear temporal shape that is similar between patients. However, the stage of disease of an individual may not be known or only partially known from what can be measured. Therefore, a latent time variable that describe individual disease stage (i.e. where the patient is along the nonlinear mean curve) can be included in the model.
Example: Modeling cognitive decline in Alzheimer's disease
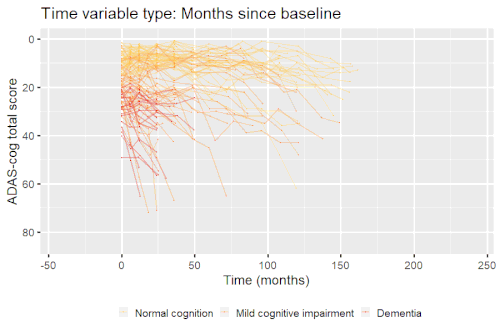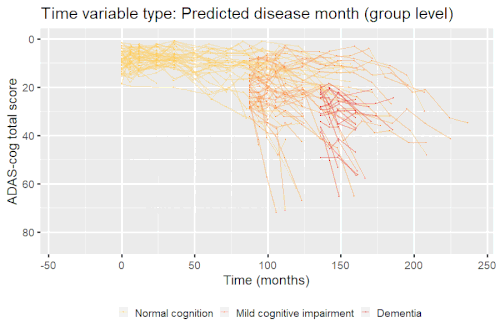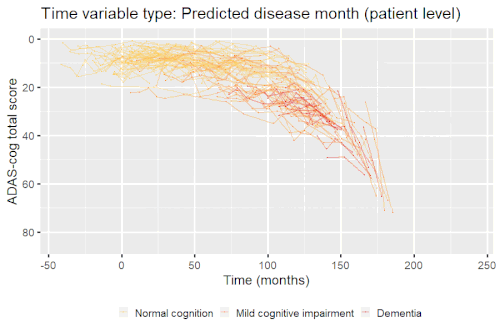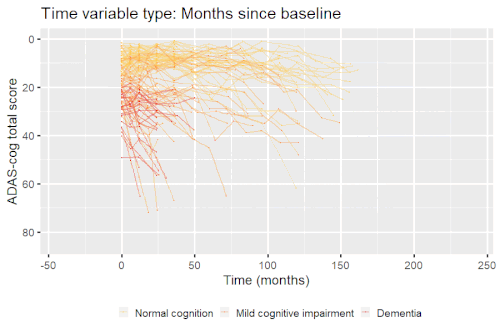
Alzheimer's disease is characterized by a progressive cognitive deterioration. However, patients may differ widely in cognitive ability and reserve, so cognitive testing at a single time point can often only be used to coarsely group individuals in different stages of disease. Now suppose we have a set of longitudinal cognitive data from individuals that are each categorized as having either normal cognition (CN), mild cognitive impairment (MCI) or dementia (DEM) at the baseline visit (time corresponding to measurement ). These longitudinal trajectories can be modeled using a nonlinear mixed effects model that allows differences in disease state based on baseline categorization:
where
- is a function that models the mean time-profile of cognitive decline whose shape is determined by the parameters ,
- represents observation time (e.g. time since baseline in the study),
- and are dummy variables that are 1 if individual has MCI or dementia at baseline and 0 otherwise,
- and are parameters that model the difference in disease progression of the MCI and dementia groups relative to the cognitively normal,
- is the difference in disease stage of individual relative to his/her baseline category, and
- is a random variable describing additive noise.
An example of such a model with an exponential mean function fitted to longitudinal measurements of the Alzheimer's Disease Assessment Scale-Cognitive Subscale (ADAS-Cog) in shown in the box. As shown, the inclusion of fixed effects of baseline categorization (MCI or dementia relative to normal cognition) and the random effect of individual continuous disease stage aligns the trajectories of cognitive deterioration to reveal a common pattern of cognitive decline.
Growth analysis
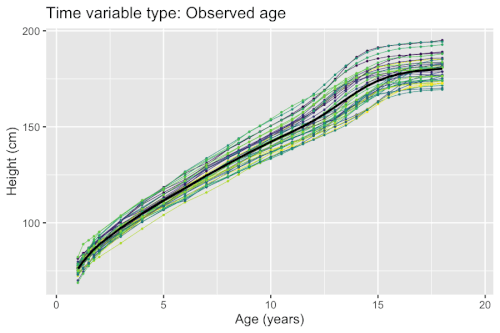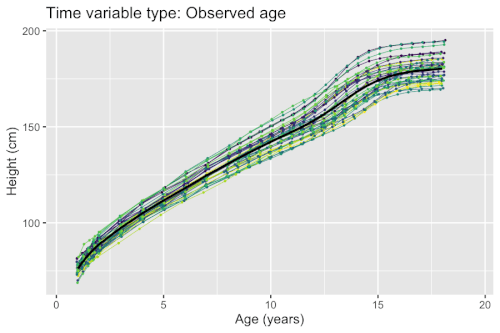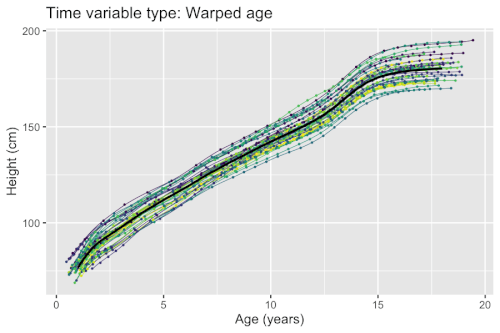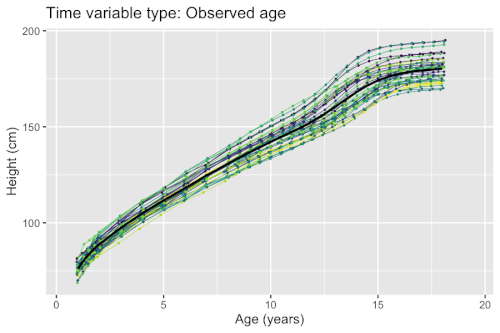
Growth phenomena often follow nonlinear patters (e.g. logistic growth, exponential growth, and hyperbolic growth). Factors such as nutrient deficiency may both directly affect the measured outcome (e.g. organisms with lack of nutrients end up smaller), but possibly also timing (e.g. organisms with lack of nutrients grow at a slower pace). If a model fails to account for the differences in timing, the estimated population-level curves may smooth out finer details due to lack of synchronization between organisms. Nonlinear mixed-effects models enable simultaneous modeling of individual differences in growth outcomes and timing.
Example: Modeling human height
Models for estimating the mean curves of human height and weight as a function of age and the natural variation around the mean are used to create growth charts. The growth of children can however become desynchronized due to both genetic and environmental factors. For example, age at onset of puberty and its associated height spurt can vary several years between adolescents. Therefore, cross-sectional studies may underestimate the magnitude of the pubertal height spurt because age is not synchronized with biological development. The differences in biological development can be modeled using random effects that describe a mapping of observed age to a latent biological age using a so-called warping function . A simple nonlinear mixed-effects model with this structure is given by
where
- is a function that represents the height development of a typical child as a function of age. Its shape is determined by the parameters ,
- is the age of child corresponding to the height measurement ,
- is a warping function that maps age to biological development to synchronize. Its shape is determined by the random effects \boldsymbol{w}_i,
- is a random variable describing additive variation (e.g. consistent differences in height between children and measurement noise).
There exists several methods and software packages for fitting such models. The so-called SITAR model[7] can fit such models using warping functions that are affine transformations of time (i.e. additive shifts in biological age and differences in rate of maturation), while the so-called pavpop model[6] can fit models with smoothly-varying warping functions. An example of the latter is shown in the box.
Pharmacokinetic/pharmacodynamic modeling

PK/PD models for describing exposure-response relationships such as the Emax model can be formulated as nonlinear mixed-effects models.[8] The mixed-model approach allows modeling of both population level and individual differences in effects that have a nonlinear effect on the observed outcomes, for example the rate at which a compound is being metabolized or distributed in the body.
See also
- Mixed model
- Fixed effects model
- Generalized linear mixed model
- Linear regression
- Mixed-design analysis of variance
- Multilevel model
- Random effects model
- Repeated measures design
References
- ^ a b Pinheiro, J; Bates, DM (2006). Mixed-effects models in S and S-PLUS. Statistics and Computing. New York: Springer Science & Business Media. doi:10.1007/b98882. ISBN 0-387-98957-9.
- ^ Bolker, BM (2008). Ecological models and data in R. Princeton University Press.
{{cite book}}:|website=ignored (help) - ^ Lindstrom, MJ; Bates, DM (1990). "Nonlinear mixed effects models for repeated measures data". Biometrics. 46 (3): 673–687. doi:10.2307/2532087. JSTOR 2532087. PMID 2242409.
- ^ Kuhn, E; Lavielle, M (2005). "Maximum likelihood estimation in nonlinear mixed effects models". Computational Statistics & Data Analysis. 49 (4): 1020–1038. doi:10.1016/j.csda.2004.07.002.
- ^ a b Raket, LL (2020). "Statistical disease progression modeling in Alzheimer's disease". Frontiers in Big Data. 3. doi:10.3389/fdata.2020.00024. S2CID 221105601.
- ^ a b Raket LL, Sommer S, Markussen B (2014). "A nonlinear mixed-effects model for simultaneous smoothing and registration of functional data". Pattern Recognition Letters. 38: 1–7. doi:10.1016/j.patrec.2013.10.018.
- ^ Cole TJ, Donaldson MD, Ben-Shlomo Y (2010). "SITAR—a useful instrument for growth curve analysis". International Journal of Epidemiology. 39 (6): 1558–66. doi:10.1093/ije/dyq115. PMC 2992626. PMID 20647267. S2CID 17816715.
- ^ Jonsson, EN; Karlsson, MO; Wade, JR (2000). "Nonlinearity detection: advantages of nonlinear mixed-effects modeling". AAPS PharmSci. 2 (3): E32. doi:10.1208/ps020332. PMC 2761142. PMID 11741248.





























