 | |||||||||||||||||||||||||||||||||
| Copper | |||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Appearance | Red-orange metallic luster | ||||||||||||||||||||||||||||||||
| Standard atomic weight Ar°(Cu) | |||||||||||||||||||||||||||||||||
| Copper in the periodic table | |||||||||||||||||||||||||||||||||
| |||||||||||||||||||||||||||||||||
| Atomic number (Z) | 29 | ||||||||||||||||||||||||||||||||
| Group | group 11 | ||||||||||||||||||||||||||||||||
| Period | period 4 | ||||||||||||||||||||||||||||||||
| Block | d-block | ||||||||||||||||||||||||||||||||
| Electron configuration | [Ar] 3d10 4s1 | ||||||||||||||||||||||||||||||||
| Electrons per shell | 2, 8, 18, 1 | ||||||||||||||||||||||||||||||||
| Physical properties | |||||||||||||||||||||||||||||||||
| Phase at STP | solid | ||||||||||||||||||||||||||||||||
| Melting point | 1357.77 K (1084.62 °C, 1984.32 °F) | ||||||||||||||||||||||||||||||||
| Boiling point | 2835 K (2562 °C, 4643 °F) | ||||||||||||||||||||||||||||||||
| Density (at 20° C) | 8.935 g/cm3 [3] | ||||||||||||||||||||||||||||||||
| when liquid (at m.p.) | 8.02 g/cm3 | ||||||||||||||||||||||||||||||||
| Heat of fusion | 13.26 kJ/mol | ||||||||||||||||||||||||||||||||
| Heat of vaporization | 300.4 kJ/mol | ||||||||||||||||||||||||||||||||
| Molar heat capacity | 24.440 J/(mol·K) | ||||||||||||||||||||||||||||||||
Vapor pressure
| |||||||||||||||||||||||||||||||||
| Atomic properties | |||||||||||||||||||||||||||||||||
| Oxidation states | −2, 0,[4] +1, +2, +3, +4 (a mildly basic oxide) | ||||||||||||||||||||||||||||||||
| Electronegativity | Pauling scale: 1.90 | ||||||||||||||||||||||||||||||||
| Ionization energies |
| ||||||||||||||||||||||||||||||||
| Atomic radius | empirical: 128 pm | ||||||||||||||||||||||||||||||||
| Covalent radius | 132±4 pm | ||||||||||||||||||||||||||||||||
| Van der Waals radius | 140 pm | ||||||||||||||||||||||||||||||||
| Other properties | |||||||||||||||||||||||||||||||||
| Natural occurrence | primordial | ||||||||||||||||||||||||||||||||
| Crystal structure | face-centered cubic (fcc) (cF4) | ||||||||||||||||||||||||||||||||
| Lattice constant | a = 361.50 pm (at 20 °C)[3] | ||||||||||||||||||||||||||||||||
| Thermal expansion | 16.64×10−6/K (at 20 °C)[3] | ||||||||||||||||||||||||||||||||
| Thermal conductivity | 401 W/(m⋅K) | ||||||||||||||||||||||||||||||||
| Electrical resistivity | 16.78 nΩ⋅m (at 20 °C) | ||||||||||||||||||||||||||||||||
| Magnetic ordering | diamagnetic[5] | ||||||||||||||||||||||||||||||||
| Molar magnetic susceptibility | −5.46×10−6 cm3/mol[6] | ||||||||||||||||||||||||||||||||
| Young's modulus | 110–128 GPa | ||||||||||||||||||||||||||||||||
| Shear modulus | 48 GPa | ||||||||||||||||||||||||||||||||
| Bulk modulus | 140 GPa | ||||||||||||||||||||||||||||||||
| Speed of sound thin rod | (annealed) 3810 m/s (at r.t.) | ||||||||||||||||||||||||||||||||
| Poisson ratio | 0.34 | ||||||||||||||||||||||||||||||||
| Mohs hardness | 3.0 | ||||||||||||||||||||||||||||||||
| Vickers hardness | 343–369 MPa | ||||||||||||||||||||||||||||||||
| Brinell hardness | 235–878 MPa | ||||||||||||||||||||||||||||||||
| CAS Number | 7440-50-8 | ||||||||||||||||||||||||||||||||
| History | |||||||||||||||||||||||||||||||||
| Naming | after Cyprus, principal mining place in Roman era (Cyprium) | ||||||||||||||||||||||||||||||||
| Discovery | Middle East (9000 BC) | ||||||||||||||||||||||||||||||||
| Symbol | "Cu": from Latin cuprum | ||||||||||||||||||||||||||||||||
| Isotopes of copper | |||||||||||||||||||||||||||||||||
| |||||||||||||||||||||||||||||||||
Copper (/[invalid input: 'icon']ˈkɒpər/ KOP-ər) is a chemical element with the symbol Cu (from Latin: cuprum) and atomic number 29. It is a ductile metal, with very high thermal and electrical conductivity. Pure copper is rather soft and malleable, and a freshly exposed surface has a reddish-orange color. It is used as a thermal conductor, an electrical conductor, a building material, and a constituent of various metal alloys.
Copper metal and alloys have been used for thousands of years. In the Roman era, copper was principally mined on Cyprus, hence the origin of the name of the metal as сyprium, "metal of Cyprus", later shortened to сuprum.
Copper compounds are commonly encountered as copper(II) salts, which often impart blue or green colors to minerals such as turquoise and have been widely used historically as pigments. Copper metal architectural structures and statuary eventually corrode to acquire a characteristic green patina. Copper as both metal and pigmented salt, has a significant presence in decorative art.
Copper(II) ions (Cu2+
) are soluble in water, where they function at low concentration as bacteriostatic substances, fungicides, and wood preservatives. In sufficient amounts, copper salts can be poisonous to higher organisms as well. However, despite universal toxicity at high concentrations, the copper(II) ion at lower concentrations is an essential trace nutrient to all higher plant and animal life.[citation needed] In animals, including humans, it is found widely in tissues, with concentration in liver, muscle, and bone. It functions as a co-factor in various enzymes and in copper-based pigments.
Characteristics
Physical


Copper occupies the same family of the periodic table as silver and gold. In terms of electronic structure, copper has one s-orbital electron on top of a filled electron shell, which forms metallic bonds.[8] Silver and gold are similar.
Copper is normally supplied, as with nearly all metals for industrial and commercial use, in a fine-grained polycrystalline form. Polycrystalline metals have greater strength than monocrystalline forms, and the difference is greater for smaller grain (crystal) sizes.[9] It is easily worked, being both ductile and malleable. The ease with which it can be drawn into wire makes it useful for electrical work, as does its high electrical conductivity.[10]
Copper has a reddish, orangish, or brownish color owing to a thin layer of tarnish (including oxides). Pure copper is pink- or peach-colored. Copper, osmium (bluish), caesium and gold (both yellow) are the only four elemental metals with a natural color other than gray or silver.[11] Copper's characteristic color results from its electronic configuration.[12]
Electrical
The comparable electron structure makes copper, silver, and gold similar in many ways: all three have high thermal and electrical conductivities, and all three are malleable. Among pure metals at room temperature, copper has the second highest electrical and thermal conductivity, after silver,[13] with a conductivity of 59.6×106 S/m. This high value is due to virtually all the valence electrons (one per atom) taking part in conduction. The resulting free electrons in copper amount to a huge charge density of 13.6×109 C/m3. This high charge density is responsible for the rather slow drift velocity of currents in copper cable, where drift velocity may be calculated as the ratio of current density to charge density. For instance, at a current density of 5×106 A/m2, the maximum current density present in household wiring and grid distribution, the drift velocity is just a little over ⅓ mm/s.[14]
During the late 20th century in the United States, the temporary popularity of aluminium for household electrical wiring resulted in many homes having a combination of copper and aluminium wiring necessitating physical contact between the two metals to provide an electrical connection. Due to galvanic corrosion, some issues were experienced by homeowners and housing contractors.[citation needed] In electronics, the purity of copper is expressed in nines: 4N for 99.99% and 6N for 99.9999%. The numeral stands for the number of nines in the purity written as a percentage; the N stands for 9. Therefore, the higher the number, the more pure the copper is.
Chemical characteristics

Copper does not react with water, but it slowly reacts with atmospheric oxygen forming a layer of brown-black copper oxide. In contrast to the oxidation of iron by wet air, this oxide layer stops the further, bulk corrosion. A green layer of copper carbonate, called verdigris, can often be seen on old copper constructions, such as the Statue of Liberty.[15]
Copper reacts with hydrogen sulfide- and sulfide-containing solutions, forming various copper sulfides on its surface. In sulfide-containing solutions, copper is less noble than hydrogen and will corrode. This is observed in everyday life when copper metal surfaces tarnish after exposure to air containing sulfur compounds.[citation needed]
Copper slowly dissolves in oxygen-containing ammonia solutions to give various water-soluble complexes with copper. Copper reacts with a combination of oxygen and hydrochloric acid to form a series of copper chlorides. Green-blue copper(II) chloride, when boiled with copper metal, undergoes comproportionation to form white copper(I) chloride.[citation needed]
Copper reacts with an acidified mixture of hydrogen peroxide to form the corresponding copper salt:
- Cu + 2 HX + H2O2 → CuX2 + 2 H2O
Isotopes
There are 29 isotopes of copper. Two of these (63Cu and 65Cu) are stable, with 63Cu comprising approximately 69% of naturally occurring copper and the other 31% being 65Cu. They both have a nuclear spin of 3/2.[16] The other isotopes are radioactive; the most stable of these is 67Cu with a half-life of 61.83 hours.[16]In addition, seven metastable isotopes have been characterized, with 68mCu the longest-lived with a half-life of 3.8 minutes. Isotopes with a mass number above 64 decay by β-, whereas those with a mass number below 64 decay by β+. 64Cu, which has a half-life of 12.7 hours, decays both ways.[17]
Two radioisotopes of copper (62Cu and 64Cu) have significant applications. 64Cu has use as a radiocontrast for X-ray imaging, and complexed with a chelate can also be used for treating cancer. 62Cu can be used in a compound (62Cu-PTSM) that functions as a radioactive tracer and is used in positron emission tomography.[18]
Occurrence

Copper can be found as native copper in mineral form (for example, in Michigan's Keweenaw Peninsula). It is a polycrystal, with the largest single crystals found to date measuring 4.4×3.2×3.2 cm.[19] Minerals such as the sulfides: chalcopyrite (CuFeS2), bornite (Cu5FeS4), covellite (CuS), chalcocite (Cu2S) are sources of copper, as are the carbonates: azurite (Cu3(CO3)2(OH)2) and malachite (Cu2CO3(OH)2) and the oxide: cuprite (Cu2O).[13]
Copper is a component in several enzymes and proteins that process oxygen. Theee include cytochrome c oxidase and certain superoxide dismutases. Most mollusks and some arthropods such as the horseshoe crab use the copper-containing pigment hemocyanin rather than iron-containing hemoglobin for oxygen transport, so their blood is blue when oxygenated rather than red.[20] Laccase and tyrosinase hydroxylate substrates, illustrated by their role in the formation of laquers.[21]
Copper also has a diverse roles in biological electron transport, the electrical "wiring" of a cell. In particular the blue copper proteins azurin and plastocyanin serve as electron relays, shuttling between copper(I) and copper(II) states. The name "blue copper" comes from their intense blue color arising from a ligand-to-metal charge transfer absorption band around 600 nm.
Production

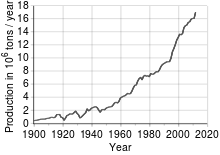


Most copper ore is mined or extracted as copper sulfides from large open pit mines in porphyry copper deposits that contain 0.4 to 1.0% copper. Examples include: Chuquicamata in Chile, Bingham Canyon Mine in Utah and El Chino Mine in New Mexico, US. The average abundance of copper found within crustal rocks is approximately 68 parts per million (ppm) by mass, and 22 ppm by atoms. In 2005, Chile was the top mine producer of copper with at least one-third world share followed by the USA, Indonesia and Peru, according to the British Geological Survey.[13]
The fraction of copper in active use is steadily increasing and the quantity available on Earth may be barely sufficient to allow all countries to reach developed world levels of copper usage.[22]
Reserves
Copper has been in use at least 10,000 years, but more than 95% of all copper ever mined and smelted has been extracted since 1900. As with many natural resources, the total amount of copper on Earth is vast (around 1014 tons just in the top kilometer of Earth's crust, or about 5 million years worth at the current rate of extraction). However, only a tiny fraction of these reserves is economically viable, given present-day prices and technologies. Various estimates of existing copper reserves available for mining vary from 25 years to 60 years, depending on core assumptions such as the growth rate.[23]
Recycling is a major source of copper in the modern world.[24] Because of these and other factors, the future of copper production and supply is the subject of much debate, including the concept of Peak copper, analogue to Peak Oil.
The price of copper, one measure of the availability of supply versus worldwide demand, has quintupled from the 60-year low in 1999, rising from US$0.60 per pound (US$1.32/kg) in June 1999 to US$3.75 per pound (US$8.27/kg) in May 2006, where it dropped to US$2.40 per pound (US$5.29/kg) in February 2007 then rebounded to US$3.50 per pound (US$7.71/kg = £3.89 = €5.00) in April 2007.[25] By early February 2009, however, weakening global demand and a steep fall in commodity prices since the previous year's highs had left copper prices at US$1.51 per pound.[26]
Methods
The concentration of copper in ores averages only 0.6%, and most commercial ores are sulfides, especially chalcopyrite (CuFeS2) and to a lesser extent chalcocite (Cu2S).[27] These minerals are concentrated from crushed ores to the level of 10-15% copper by froth flotation. Heating these materials with silica in the process called flash smelting removes much of the iron as slag. The process exploits the greater ease of converting iron sulfides into its oxides, which in turn react with the silica to form the silicate slag, which floats on top of the heated mass. The resulting copper matte, consisting of Cu2S is then roasted to convert all sulfides into oxides:[27]
The cuprous oxide is converted to blister copper upon heating:
- 2 Cu2O → 4 Cu + O2
The latter step exploits the relatively easy reduction of copper oxides to copper metal. Natural gas is blown across the blister to remove most of the remaining oxygen and electrorefining is performed on the resulting material to produce pure copper:
- Cu2+ + 2 e- → Cu
Recycling
Copper, like aluminium, is 100% recyclable without any loss of quality whether in a raw state or contained in a manufactured product. In volume, copper is the third most recycled metal after iron and aluminium. It is estimated that 80% of the copper ever mined is still in use today.[28]
High purity copper scrap is directly melted in a furnace and the molten copper is deoxidized and cast into billets, or ingots. Lower purity scrap is usually refined by electroplating process. In this process, the scrap is dissolved into a bath of sulfuric acid, and the copper then electroplated out of the solution.[29]
Compounds

Copper forms a rich variety of compounds with oxidation states +1 and +2, which are often called cuprous and cupric, respectively.[30]
Binary compounds
As for other elements, the simplest compounds of copper are binary compounds, i.e. those containing only two elements. The principal ones are the oxides, sulfides and halides. Both cuprous and cupric oxides are known: Copper(I) oxide (cuprite) and copper(II) oxide (tenorite). Among the numerous copper sulfides, important examples include copper(I) sulfide (chalcocite) and copper(II) sulfide (covellite).
Three cuprous halides are well known: chloride, bromide, and iodide are known, as are three cupric halides copper(II) fluoride, chloride, and bromide. Attempts to prepare copper(II) iodide give cuprous iodide and iodine.[30]
- 2 Cu2+ + 4 I− → 2 CuI + I2
Coordination chemistry

Copper, like all metals, form coordination complexes with ligands. In aqueous solution, copper(II) exists as [Cu(H2O)6]2+. This complex exhibits the fastest water exchange rate (speed of water ligands breaking away and re-attaching) for any transition metal aquo complex. Adding an aqueous solution of sodium hydroxide will cause the precipitation of light blue solid copper(II) hydroxide. A simplified equation is:
- Cu2+ + 2 OH− → Cu(OH)2
An aqueous ammonia causes the same precipitate to form. Upon adding excess ammonia, the precipitate dissolves, forming a deep blue ammonia complex, tetraamminecopper(II):
- Cu(H2O)4(OH)2 + 4 NH3 → [Cu(H2O)2(NH3)4]2+ + 2 H2O + 2 OH−
Many other oxyanions form complexes, these include copper(II) acetate, copper(II) nitrate, and copper(II) carbonate. The carbonate is a component of the green tarnish found on copper-clad roofs or domes on older buildings. Copper(II) sulfate forms a blue crystalline pentahydrate, which is the most familiar copper compound in the laboratory. It is used as a fungicide called the Bordeaux mixture.[31]

Polyols, compounds containing more than one alcohol, often interact with cupric salts. Traditionally, copper salts were used to test for reducing sugars. Specifically, using Benedict's reagent and Fehling's solution the presence of the sugar is signaled by a color change from blue Cu(II) to reddish copper(I) oxide (Cu2O).[32] Ammonia complexes of cupric hydroxide, Schweizer's reagent, and related complex with ethylenediamine and other amines dissolve cellulose (a polymeric derivative of the polyol glucose). [33] Amino acids also form very stable chelate complexes with copper(II).
Many wet-chemical tests for copper ions exist, one involving potassium ferrocyanide, which gives a brown precipitate with copper salts.
Organocopper chemistry
Compounds that contain a carbon-copper bond are known as organocopper compounds. Although they are very reactive towards oxygen to form copper(I) oxide, they have many uses in chemistry. They are synthesized by treating copper(I) compounds with Grignard reagents, terminal alkynes or organolithium reagents.[34] In particular, the last reaction described produces a Gilman reagent. Gilman reagents can undergo substitution with alkyl halides to form coupling products. As such, they are important in the field of organic synthesis. Copper(I) acetylide is highly shock-sensitive but is an intermediate in reactions such as the Cadiot-Chodkiewicz coupling and the Sonogashira coupling.[citation needed] Conjugate addition to enones[35] and carbocupration of alkynes[36] can also be achieved with organocopper compounds.
Copper(I) forms a variety of weak complexes with alkenes and carbon monoxide, especially in the presence of amine ligands.[37]
Copper(III) and copper(IV)
Complexes of cpper(III) are frequent intermediates in reactions of organocopper compounds. Dicopper oxo complexes also feature copper(III).[38] Fluoride ligands, being highly basic, often stabilize metal ions in high oxidation states; indeed, representative copper(III) and even copper(IV) complex are fluorides. These include K3CuF6 and Cs2CuF6.[30] With di- and tripeptides, purple-colored complexes of copper(III) have been observed, this high oxidation state being stabilized by the deprotonated amide ligands.[39]
History
Copper Age
Copper, as native copper, is one of the few metals to occur naturally. Copper was known to some of the oldest civilizations on record, and has a history of use that is at least 10,000 years old. Some estimates of copper's discovery place this event around 9000 BC in the Middle East.[40] A copper pendant was found in what is now northern Iraq that dates to 8700 BC.[41] It is probable that gold and meteoritic iron were the only metals used by humans before copper.[42] By 5000 BC, there are signs of copper smelting: the refining of copper from simple copper compounds such as malachite or azurite. Among archaeological sites in Anatolia, Çatal Höyük (~6000 BC) features native copper artifacts and smelted lead beads, but no smelted copper. Can Hasan (~5000 BC) had access to smelted copper but the oldest smelted copper artifact found (a copper chisel from the chalcolithic site of Prokuplje in Serbia) has pre-dated Can Hasan by 500 years. The smelting facilities in the Balkans appear to be more advanced than the Anatolian forges found at a later date, so it is quite probable that copper smelting originated in the Balkans. Investment casting was realized in 4500–4000 BC in Southeast Asia.[40] Carbon dates have established mining at around 2280 to 1890 BC at Alderley Edge in Cheshire, UK.[43]

Copper smelting appears to have been developed independently in several parts of the world. In addition to its development in the Balkans by 5500 BC, it was developed in China before 2800 BC, in the Andes around 2000 BC, in Central America around 600 AD, and in West Africa around 900 AD.[44] Copper is found extensively in the Indus Valley Civilization by the 3rd millennium BC. In Europe, Ötzi the Iceman, a well-preserved male dated to 3300–3200 BC, was found with an axe with a copper head 99.7% pure. High levels of arsenic in his hair suggest he was involved in copper smelting. Over the course of centuries, experience with copper has assisted the development of other metals; for example, knowledge of copper smelting led to the discovery of iron smelting.[citation needed]
In the Americas production in the Old Copper Complex, located in present day Michigan and Wisconsin, was dated to between 6000 to 3000 BC.[45][46]
Bronze Age
Alloying of copper with zinc or tin to make brass or bronze was practiced soon after the discovery of copper. Copper and bronze artifacts from Sumerian cities date to 3000 BC,[47] and Egyptian artifacts of copper and copper-tin alloys nearly as old. The use of bronze became so widespread in Europe approximately from 2500 BC to 600 BC that it has been named the Bronze Age. The transitional period in certain regions between the preceding Neolithic period and the Bronze Age is termed the Chalcolithic ("copper-stone"), with some high-purity copper tools being used alongside stone tools. Brass (copper-zinc alloy) was known to the Greeks, but only became a significant supplement to bronze during the Roman empire.[47]
Antiquity and Middle Ages


In Greek, the metal was known by the name chalkos (χαλκός). Copper was a very important resource for the Romans, Greeks and other ancient peoples. In Roman times, it became known as aes Cyprium (aes being the generic Latin term for copper alloys such as bronze and other metals, and Cyprium because so much of it was mined in Cyprus). From this, the phrase was simplified to cuprum, hence the English copper. Copper was associated with the goddess Aphrodite/Venus in mythology and alchemy, owing to its lustrous beauty, its ancient use in producing mirrors, and its association with Cyprus, which was sacred to the goddess. In astrology and alchemy the seven heavenly bodies known to the ancients were associated with seven metals also known in antiquity, and Venus was assigned to copper.[48]
Britain's first use of brass occurred around the 3rd–2nd century BC. In North America, copper mining began with marginal workings by Native Americans. Native copper is known to have been extracted from sites on Isle Royale with primitive stone tools between 800 and 1600.[49]
Copper metallurgy was flourishing in South America, particularly in Peru around the beginning of the first millennium AD. Copper technology proceeded at a much slower rate on other continents. Africa's major location for copper reserves is Zambia. Copper burial ornamentals dated from the 15th century have been uncovered, but the metal's commercial production did not start until the early 20th century. Australian copper artifacts exist, but they appear only after the arrival of the Europeans; the aboriginal culture apparently did not develop their own metallurgical abilities.[citation needed]
Crucial in the metallurgical and technological worlds, copper has also played an important cultural role, particularly in currency. Romans in the 6th through 3rd centuries BC used copper lumps as money. At first, just the copper itself was valued, but gradually the shape and look of the copper became more important. Julius Caesar had his own coins, made from a copper-zinc alloy, while Octavianus Augustus Caesar's coins were made from Cu-Pb-Sn alloys. With an estimated annual output of around 15,000 t, Roman copper mining and smelting activities reached a scale unsurpassed until the time of the Industrial Revolution; the provinces most intensely mined were those of Hispania, Cyprus and in Central Europe.[50][51]
The gates of the Temple of Jerusalem used Corinthian bronze made by depletion gilding. Corinthian bronze was most prevalent in Alexandria, where alchemy is thought to have begun.[52] In ancient India (before 1000 BC), copper was used in the holistic medical science Ayurveda for surgical instruments and other medical equipment. Ancient Egyptians (~2400 BC) used copper for sterilizing wounds and drinking water, and as time passed, (~1500 BC) for headaches, burns, and itching. Hippocrates (~400 BC) used copper to treat leg ulcers associated with varicose veins. Ancient Aztecs fought sore throats by gargling with copper mixtures.[citation needed]
Copper is also the part of many rich stories and legends, such as that of Iraq's Baghdad Battery. Copper cylinders soldered to lead, which date back to 248 BC to 226 AD, resemble a galvanic cell, leading people to believe this may have been the first battery. This claim has so far not been substantiated.[citation needed]
The Bible also refers to the importance of copper: "Men know how to mine silver and refine gold, to dig iron from the earth and melt copper from stone" (Job 28:1–2).[citation needed]
Modern period

The Great Copper Mountain was a mine in Falun, Sweden, that operated for a millennium from the 10th century to 1992. It produced as much as two thirds of Europe's copper needs in the 17th century and helped fund many of Sweden's wars during that time.[53] It was referred to as the nation's treasury; Sweden had a copper backed currency.[citation needed]
Throughout history, copper's use in art has extended far beyond currency. It was used by Renaissance sculptors, in pre-photographic technology known as the daguerreotype, and the Statue of Liberty. Copper plating and Copper sheathing for ships' hulls was widespread. The ships of Christopher Columbus were among the earliest to have this protection.[54] The Norddeutsche Affinerie in Hamburg was the first modern electroplating plant starting its production in 1876.[55] The German scientist Gottfried Osann invented powder metallurgy of copper in 1830 while determining the metal's atomic mass. Around then it was also discovered that the amount and type of alloying element (e.g. tin) would affect the tones of bells, leading to bell casting. Flash smelting was developed by Outokumpu in Finland and first applied at the Harjavalta plant in 1949. The energy-efficient process accounts for 50% of the world’s primary copper production.[56]
Copper mines have been pivotal in some economic and sociological disputes. The 1906 Cananea Strike in Mexico dealt with issues of work organization. The Teniente copper mine (1904–1951) raised political issues about capitalism and class structure. Japan's largest copper mine, the Ashio mine, was the site of a riot in 1907. The Arizona miners' strike of 1938 dealt with American labor issues including the "right to strike".[citation needed]
The Intergovernmental Council of Copper Exporting Countries, defunct since 1992, once tried to play a similar role for copper as OPEC does for oil, but never achieved the same influence, not least because the second-largest producer, the United States, was never a member. Formed in 1967, its principal members were Chile, Peru, Zaire, and Zambia.[57]
Applications

About 2% of the copper production is used for the production of compounds; the main applications are for nutritional supplements and fungicides in agriculture. The remaining 98% of the copper supply is used as the metal, taking advantage of distinctive physical properties – being malleable and ductile, a good conductor of both heat and electricity, and being resistant to corrosion.[31]
In contact with metals of different electric potential, such as a copper pipe joined to an iron pipe, the completion of an electrical circuit will cause the juncture to act as an electrochemical cell; much like a single cell of a battery. This can occur, for example, through the common ground and is particularly true in the presence of moisture. The weak electrical currents themselves are harmless but the electrochemical reaction will cause the conversion of the iron to other compounds, eventually destroying the functionality of the union.[citation needed]
Copper is often too soft for its applications, so it is incorporated in numerous alloys. For example, brass is a copper-zinc alloy, and bronze is a copper-tin alloy.[58]
Copper can be machined, although it is usually necessary to use an alloy for intricate parts, such as threaded components, to get good machinability characteristics. Good thermal conduction makes it useful for heat sinks and in heat exchangers. It is widely used in piping for water supplies, refrigeration and air conditioning.[citation needed]

Copper's electrical properties are exploited in its use as copper wire, electromagnets, electrical relays, busbars and switches. Integrated circuits and printed circuit boards increasingly feature copper in place of aluminium because of its superior electrical conductivity. As a material in the manufacture of computer heat sinks, as a result of its superior heat dissipation capacity to aluminium. Vacuum tubes, cathode ray tubes, and the magnetrons in microwave ovens use copper, as do wave guides for microwave radiation.[citation needed]
Architecture and industry

Due to the waterproof nature of copper, it has been used as the roofing material of many buildings since ancient times. The green colour on these buildings is due to a long-term chemical reaction: copper is first oxidised to copper oxide, then to cuprous and cupric sulfide and finally to copper carbonate, also called verdigris, which is highly corrosion-resistant.[59]
While electrical applications use oxygen-free copper, unalloyed copper used in architectural applications is the lower-purity phosphorus deoxidized copper (also called Cu-DHP).[60]
Copper is also used in statuary, coinage (as cupronickel), and shipbuilding (as Monel). It was also used in Watt's steam engine firebox due to copper's high thermal conductivity.[citation needed]

Copper is used in lightning rods. High above the roof, copper spikes are connected to a very thick copper cable which leads to a large metal plate underneath the ground. The electric current is dispersed throughout the ground harmlessly, instead of destroying the main structure.[61] Copper is also used in lead-free solder, as alloyed with tin[62]; there are many types, some also alloyed with silver.[63]
Copper has good corrosion resistance, but not as good as gold's. It has excellent brazing and soldering properties and can also be welded; the best results are obtained with gas metal arc welding.[64]
Copper in alloys
Numerous copper alloys exist, many with important historical and contemporary uses. Speculum metal and bronze are alloys of copper and tin. Brass is an alloy of copper and zinc. Monel metal, also called cupronickel, is an alloy of copper and nickel. While the metal bronze usually refers to copper-tin alloys, it also is a generic term for any alloy of copper, such as aluminium bronze, silicon bronze, and manganese bronze. Copper is one of the most important constituents of carat silver and gold alloys and carat solders used in the jewelry industry, modifying the color, hardness and melting point of the resulting alloys.[65]
Antibiofouling applications
Copper has long been used as a biostatic surface to line parts of ships to protect against barnacles and mussels. It was originally used pure, but has since been superseded by Muntz metal. Bacteria will not grow on a copper surface because it is biostatic. Similarly, as discussed in copper alloys in aquaculture, copper alloys have become important netting materials in the aquaculture industry for the fact that they are antimicrobial and prevent biofouling even in extreme conditions[66] and have strong structural and corrosion-resistant[67] properties in marine environments. It is the combination of these properties – antifouling, high strength, and corrosion resistance – that has made copper alloys a desirable material for netting and structural materials in commercial large-scale fish farming operations.[citation needed]
Copper doorknobs are used by hospitals to reduce the transfer of disease, and Legionnaires' disease is suppressed by copper tubing in air-conditioning systems.[citation needed]
Other uses
Copper compounds in liquid form are used as a wood preservative, particularly in treating original portion of structures during restoration of damage due to dry rot. Together with zinc, copper wires may be placed over non-conductive roofing materials to discourage the growth of moss. Copper(II) sulfate is used as a fungicide and as algae control in domestic lakes and ponds. It is used in gardening powders and sprays to kill mildew.[31] Copper has been used in textile fibers to create antimicrobial protective fabrics.[68]
Copper has been used as a component in ceramic glazes and stained glass and in musical instruments like brass instruments and timpanis. It has also been used in Class D fire extinguishers as powder to extinguish lithium fires by covering the burning metal and acting as a heat sink.[citation needed]
Copper has been used in weaponry, like in small arms ammunition with copper as a jacket, a casing material for bullets as brass and a liner in shaped charge armor-piercing warheads and demolition explosives.[citation needed]
Copper is frequently used in electroplating, usually as a base for other metals such as nickel. It is also commonly found in jewellery and folklore states that copper bracelets relieve arthritis symptoms, though this is not proven.[citation needed]
Nutrition

Copper is an essential trace element in all living things. The human body contains copper at a level of about 1.4 to 2.1 mg for each kg of body mass.[69] Copper is distributed widely in the body and occurs in liver, muscle and bone. Copper is transported in the bloodstream on a plasma protein called ceruloplasmin. When copper is first absorbed in the gut it is transported to the liver bound to albumin. Copper metabolism and excretion is controlled delivery of copper to the liver by ceruloplasmin, where it is excreted in bile.[citation needed]
Daily dietary standards for copper have been set by various health agencies around the world. Researchers specializing in the fields of microbiology, toxicology, nutrition, and health risk assessments are working together to define precise copper levels required for essentiality while avoiding deficient or excess copper intakes.[citation needed]
The RDA for copper in normal healthy adults is 0.9 mg/day; however, professional research on the subject recommends 3.0 mg/day.[70] Because of its role in facilitating iron uptake, copper deficiency can often produce anemia-like symptoms. Conversely, an accumulation of copper in body tissues causes the symptoms of Wilson's disease in humans. Copper deficiency is also associated with neutropenia, bone abnormalities, hypopigmentation, impaired growth, increased incidence of infections, and abnormalities in glucose and cholesterol metabolism. Severe deficiency can be found by testing for low plasma or serum copper levels, low ceruloplasmin, and low red blood cell superoxide dismutase (SOD) levels. However, these tests are not sensitive to marginal copper status. The "cytochrome c oxidase activity of leucocytes and platelets" has been stated as another factor in deficiency, but the results have not been confirmed by replication.[71]
Chronic copper depletion leads to abnormalities in metabolism of fats, high triglycerides, non-alcoholic steatohepatitis (NASH), fatty liver disease and poor melanin and dopamine synthesis causing depression and sunburn.[citation needed]
Antimicrobial properties
Copper, like silver, is antibacterial via the oligodynamic effect. For example, brass doorknobs disinfect themselves of much bacteria within a period of eight hours.[72] It is effective against methicillin-resistant Staphylococcus aureus (MRSA),[73] Escherichia coli[74] and other pathogens.[75][76][77] At lower temperatures, longer times are required to kill bacteria.
On February 29, 2008, the United States EPA registered 275 alloys, containing greater than 65% nominal copper content, as antimicrobial materials.[78] Registered alloys include pure copper, an assortment of brasses and bronzes, and additional alloys. EPA-sanctioned tests using Good Laboratory Practices were conducted in order to obtain several antimicrobial claims valid against MRSA, Enterobacter aerogenes, Escherichia coli O157: H7 and Pseudomonas aeruginosa. The EPA registration allows the manufacturers of these copper alloys to legally make public health claims as to the health effects of these materials. Several of the aforementioned bacteria are responsible for a large portion of the nearly two million hospital-acquired infections contracted each year in the United States"[79] frequently touched surfaces harbor bacteria and increase the risk for contracting infections, and thus covering these with copper alloys helps reduce the risk.
Precautions
Even though copper is essential to life, ingesting too much of it can lead to health problems - for example from eating acidic food that has been cooked with copper cookware. The reason for this is because free copper is a generator of reactive oxygen species that can damage DNA and other biomolecules.[citation needed] Specific examples of this include Wilson's disease and Alzheimer's disease, with symptoms including vomiting, jaundice and cirrhosis, of which the last has been linked to boiling milk in copper cookware. The Merck Manual of Diagnosis and Therapy states that a genetic defect is associated with this cirrhosis.[80] The toxicity can be treated with heavy metal chelating agents like dimercaprol. Since copper is actively excreted by the body, chronic copper toxicity in humans without the genetic defect in handling copper has not been demonstrated.[69] However, gram quantities of copper salts taken in suicide attempts have produced acute copper toxicity in normal humans. Equivalent amounts of copper salts (30 mg/kg) are toxic in animals.[81]
See also
- Electroplating
- Erosion corrosion of copper water tubes
- Metal theft
- Smelter
- Peak copper
- Category:Copper mining companies
References
- ^ "Standard Atomic Weights: Copper". CIAAW. 1969.
- ^ Prohaska, Thomas; Irrgeher, Johanna; Benefield, Jacqueline; Böhlke, John K.; Chesson, Lesley A.; Coplen, Tyler B.; Ding, Tiping; Dunn, Philip J. H.; Gröning, Manfred; Holden, Norman E.; Meijer, Harro A. J. (2022-05-04). "Standard atomic weights of the elements 2021 (IUPAC Technical Report)". Pure and Applied Chemistry. doi:10.1515/pac-2019-0603. ISSN 1365-3075.
- ^ a b c Arblaster, John W. (2018). Selected Values of the Crystallographic Properties of Elements. Materials Park, Ohio: ASM International. ISBN 978-1-62708-155-9.
- ^ Moret, Marc-Etienne; Zhang, Limei; Peters, Jonas C. (2013). "A Polar Copper–Boron One-Electron σ-Bond". J. Am. Chem. Soc. 135 (10): 3792–3795. doi:10.1021/ja4006578. PMID 23418750.
- ^ Lide, D. R., ed. (2005). "Magnetic susceptibility of the elements and inorganic compounds". CRC Handbook of Chemistry and Physics (PDF) (86th ed.). Boca Raton (FL): CRC Press. ISBN 0-8493-0486-5. Archived from the original (PDF) on 2011-03-03.
- ^ Weast, Robert (1984). CRC, Handbook of Chemistry and Physics. Boca Raton, Florida: Chemical Rubber Company Publishing. pp. E110. ISBN 0-8493-0464-4.
- ^ Kondev, F. G.; Wang, M.; Huang, W. J.; Naimi, S.; Audi, G. (2021). "The NUBASE2020 evaluation of nuclear properties" (PDF). Chinese Physics C. 45 (3): 030001. doi:10.1088/1674-1137/abddae.
- ^ "WebElements Periodic Table of the Elements | Copper | ionization energies data". WebElements. Retrieved 2011-04-08.
- ^ Smith, William F. and Hashemi, Javad (2003). Foundations of Materials Science and Engineering. McGraw-Hill Professional. p. 223. ISBN 0072921943.
{{cite book}}: CS1 maint: multiple names: authors list (link) - ^ "WebElements Periodic Table of the Elements | Copper | uses". WebElements. Retrieved 2011-04-08.
- ^ Chambers, William; Chambers, Robert (1884). Chambers's Information for the People. Vol. L (5th ed.). W. & R. Chambers. p. 312. ISBN 0665469128.
- ^ Copper is an exception to Madelung's rule, with only one electron in the 4s subshell instead of two. The energy of a photon of blue or violet light is sufficient for a d shell electron to absorb it and transition to the half-full s shell. Thus, the light reflected by copper is missing some blue/violet components and appears red. This phenomenon is also exhibited by gold which has a corresponding 5d10 6s1 structure. Razeghi, M. (2006). Fundamentals of Solid State Engineering. Birkhäuser. pp. 154–156. ISBN 0387281525.
- ^ a b c Hammond, C. R. (2004). The Elements, in Handbook of Chemistry and Physics 81st edition. CRC press. ISBN 0849304857.
- ^ Seymour, J. (1972). Physical Electronics. Pitman Publishing. pp. 25–27, 53–54. ISBN 0273411764.
- ^ "Copper.org: Education: Statue of Liberty: Reclothing the First Lady of Metals – Repair Concerns". Copper.org. Retrieved 11 April 2011.
- ^ a b Audi, G (2003). "Nubase2003 Evaluation of Nuclear and Decay Properties". Nuclear Physics A. 729. Atomic Mass Data Center: 3. doi:10.1016/j.nuclphysa.2003.11.001.
- ^ "Interactive Chart of Nuclides". National Nuclear Data Center. Retrieved 2011-04-08.
- ^ Okazawa, Hidehiko; Yonekura, Yoshiharu; Fujibayashi, Yasuhisa; et al. (1994). "Clinical Application and Quantitative Evaluation of Generator-Produced Copper-62-PTSM as a Brain Perfusion Tracer for PET" (PDF). Journal of Nuclear Medicine. 35 (12): 1910–1915.
{{cite journal}}: Unknown parameter|month=ignored (help) - ^ Rickwood, P. C. (1981). "The largest crystals" (PDF). American Mineralogist. 66: 885.
- ^ "Fun Facts". Horseshoe Crab. University of Delaware. Retrieved 2008-07-13.
- ^ S. J. Lippard, J. M. Berg “Principles of Bioinorganic Chemistry” University Science Books: Mill Valley, CA; 1994. ISBN 0-935702-73-3.
- ^ Gordon, R. B.; Bertram, M.; Graedel, T. E. (2006). "Metal stocks and sustainability". PNAS. 103 (5): 1209–1214. doi:10.1073/pnas.0509498103. PMC 1360560. PMID 16432205.
- ^ Brown, Lester (2006). Plan B 2.0: Rescuing a Planet Under Stress and a Civilization in Trouble. New York: W.W. Norton. p. 109. ISBN 0393328317.
- ^ Leonard, Andrew (2006-03-02). "Peak copper?". Salon – How the World Works. Retrieved 2008-03-23.
- ^ "Copper Trends: Live Metal Spot Prices".
- ^ Ackerman, R. (02-04-2009). "A Bottom In Sight For Copper". Forbes.
{{cite news}}: Check date values in:|date=(help) - ^ a b Greenwood, Norman N.; Earnshaw, Alan (1997). Chemistry of the Elements (2nd ed.). Butterworth-Heinemann. ISBN 978-0-08-037941-8.
- ^ "International Copper Association".
- ^ "Overview of Recycled Copper" Copper.org.
- ^ a b c Holleman, A. F.; Wilburg, E. (2001). Inorganic Chemistry. San Diego: Academic Press. ISBN 978-0-12-352651-9.
- ^ a b c Wiley-Vch, (2007-04-02). "Nonsystematic (Contact) Fungicides". Ullmann's Agrochemicals. p. 623. ISBN 9783527316045.
{{cite book}}: CS1 maint: extra punctuation (link) - ^ Ralph L. Shriner, Christine K. F. Hermann, Terence C. Morrill, David Y. Curtin, Reynold C. Fuson "The Systematic Identification of Organic Compounds" 8th edition, J. Wiley, Hoboken. ISBN 10 0-471-21503-1
- ^ Kay Saalwa1chter, Walther Burchard, Peter Klüfers, G. Kettenbach, and Peter Mayer, Dieter Klemm, Saran Dugarmaa "Cellulose Solutions in Water Containing Metal Complexes" Macromolecules 2000, 33, 4094-4107
- ^ "Modern Organocopper Chemistry" Norbert Krause, Ed., Wiley-VCH, Weinheim, 2002. ISBN: 9783527297733.
- ^ "An Addition of an Ethylcopper Complex to 1-Octyne: (E)-5-Ethyl-1,4-Undecadiene" (PDF). Organic Syntheses. 64: 1. 1986.
- ^ Kharasch, M. S.; Tawney, P. O. (1941). "Factors Determining the Course and Mechanisms of Grignard Reactions. II. The Effect of Metallic Compounds on the Reaction between Isophorone and Methylmagnesium Bromide". Journal of the American Chemical Society. 63 (9): 2308–2316. doi:10.1021/ja01854a005.
{{cite journal}}: Unknown parameter|month=ignored (help) - ^ Sadako Imai, Kiyoshi Fujisawa, Takako Kobayashi, Nobuhiko Shirasawa, Hiroshi Fujii, Tetsuhiko Yoshimura, Nobumasa Kitajima, and Yoshihiko Moro-oka "63Cu NMR Study of Copper(I) Carbonyl Complexes with Various Hydrotris(pyrazolyl)borates: Correlation between 63Cu Chemical Shifts and CO Stretching Vibrations" Inorg. Chem., 1998, volume 37, pp 3066–3070.doi:10.1021/ic970138r
- ^ Lewis, E. A.; Tolman, W. B. (2004). "Reactivity of Dioxygen-Copper Systems". Chemical Reviews. 104: 1047–1076. doi:10.1021/cr020633r.
- ^ McDonald, M. R.; Fredericks, F. C.; Margerum, D. W. (1997). "Characterization of Copper(III)-Tetrapeptide Complexes with Histidine as the Third Residue". Inorganic Chemistry. 36: 3119–3124.
- ^ a b "CSA – Discovery Guides, A Brief History of Copper". Csa.com. Retrieved 2008-09-12.
- ^ Rayner W. Hesse (2007). Rayner W. Hesse. Greenwood Publishing Group. p. 56. ISBN 0313335079.
- ^ "Copper". Elements.vanderkrogt.net. Retrieved 2008-09-12.
- ^ Timberlake, S. and Prag A.J.N.W. (2005). The Archaeology of Alderley Edge: Survey, excavation and experiment in an ancient mining landscape. Oxford: John and Erica Hedges Ltd. p. 396.
- ^ Cowen, R. "Essays on Geology, History, and People, Chapter 3: "Fire and Metals: Copper". Retrieved 2009-07-07.
- ^ Pleger, Thomas C. "A Brief Introduction to the Old Copper Complex of the Western Great Lakes: 4000–1000 BC", Proceedings of the Twenty-seventh Annual Meeting of the Forest History Association of Wisconsin, Oconto, Wisconsin, October 5, 2002, pp. 10–18.
- ^ Emerson, Thomas E. and McElrath, Dale L. Archaic Societies: Diversity and Complexity Across the Midcontinent, SUNY Press, 2009 ISBN 1-4384-2701-8.
- ^ a b McNeil, Ian (2002). Encyclopaedia of the History of Technology. London ; New York: Routledge. pp. 13, 48–66. ISBN 0203192117.
- ^ Rickard, T. A. (1932). "The Nomenclature of Copper and its Alloys". The Journal of the Royal Anthropological Institute of Great Britain and Ireland. 62. The Journal of the Royal Anthropological Institute of Great Britain and Ireland, Vol. 62: 281. doi:10.2307/2843960. JSTOR 2843960.
- ^ Martin, Susan R. (1995). "The State of Our Knowledge About Ancient Copper Mining in Michigan". The Michigan Archaeologist. 41 (2–3): 119.
- ^ Hong, S.; Candelone, J.-P.; Patterson, C. C.; Boutron, C. F. (1996). "History of Ancient Copper Smelting Pollution During Roman and Medieval Times Recorded in Greenland Ice". Science. 272 (5259): 246–249 (247f.). doi:10.1126/science.272.5259.246.
- ^ de Callataÿ, François (2005). "The Graeco-Roman Economy in the Super Long-Run: Lead, Copper, and Shipwrecks". Journal of Roman Archaeology. 18: 361–372 (366–369).
- ^ Jacobson, D. M.; Warman, John M.; Barentsen, Helma M.; van Dijk, Marinus; Zuilhof, Han; Sudhölter, Ernst J. R. (2000). "Corinthian Bronze and the Gold of the Alchemists" (PDF). Macromolecules. 33 (2): 60. Bibcode:2000MaMol..33...60S. doi:10.1021/ma9904870.
- ^ Lynch, Martin (2004-04-15). Mining in World History. p. 60. ISBN 9781861891730.
- ^ "Copper History". Retrieved 2008-09-04.
- ^ Stelter, M.; Bombach, H. (2004). "Process Optimization in Copper Electrorefining". Advanced Engineering Materials. 6 (7): 558. doi:10.1002/adem.200400403.
- ^ "Outokumpu Flash Smelting" (PDF). Outokumpu. p. 2.
- ^ Karen A. Mingst (1976). "Cooperation or illusion: an examination of the intergovernmental council of copper exporting countries". International Organization. 30 (2): 263–287. doi:10.1017/S0020818300018270.
- ^ "Copper". American Elements. 2008. Retrieved 2008-07-12.
- ^ Berg, Jan. "Why did we paint the library's roof?". Archived from the original on 2007-06-25. Retrieved 2007-09-20.
- ^ ASTM B 152, Standard Specification for Copper Sheet, Strip, Plate, and Rolled Bar.
- ^ Physics 1, Jacaranda Science. 3rd Ed. 2009.
- ^ Balver Zinn Solder Sn97Cu3
- ^ Balver Zinn Solder SCA (SnCu0.7Ag0.3)
- ^ Davis, Joseph R. (2001). Copper and Copper Alloys. ASM International. pp. 3–6, 266. ISBN 0871707268.
- ^ "Gold Jewellery Alloys". World Gold Council. Retrieved 2009-06-06.
- ^ Edding, Mario E., Flores, Hector, and Miranda, Claudio, (1995), Experimental Usage of Copper-Nickel Alloy Mesh in Mariculture. Part 1: Feasibility of usage in a temperate zone; Part 2: Demonstration of usage in a cold zone; Final report to the International Copper Association Ltd.
- ^ Corrosion Behaviour of Copper Alloys used in Marine Aquaculture
- ^ "Antimicrobial Products that Shield Against Bacteria and Fungi". Cupron, Inc. 2008. Retrieved 2008-07-13.
- ^ a b "Amount of copper in the normal human body, and other nutritional copper facts". Retrieved April 3, 2009.
- ^ Copper. In: Recommended Dietary Allowances. Washington, D.C.: National Research Council, Food Nutrition Board, NRC/NAS. 1980. pp. 151–154.
- ^ Bonham, M.; et al. (2002). "The immune system as a physiological indicator of marginal copper status?". British Journal of Nutrition. 87 (5): 393–403. doi:10.1079/BJN2002558. PMID 12010579.
{{cite journal}}: Explicit use of et al. in:|author=(help) - ^ Kuhn, P. J. (1983). "Doorknobs: A Source of Nosocomial Infection?". Retrieved 2007-08-15.
- ^ Noyce JO, Michels H, Keevil CW (2006). "Potential use of copper surfaces to reduce survival of epidemic meticillin-resistant Staphylococcus aureus in the healthcare environment". J. Hosp. Infect. 63 (3): 289. doi:10.1016/j.jhin.2005.12.008. PMID 16650507.
{{cite journal}}: CS1 maint: multiple names: authors list (link) - ^ Noyce, J. O.; Michels, H. and Keevil C. W (2006). "Use of copper cast alloys to control Escherichia coli O157 cross-contamination during food processing". Appl. Environ. Microbiol. 72 (6): 4239. doi:10.1128/AEM.02532-05. PMC 1489622. PMID 16751537.
{{cite journal}}: CS1 maint: multiple names: authors list (link) - ^ Mehtar S, Wiid I, Todorov SD (2008). "The antimicrobial activity of copper and copper alloys against nosocomial pathogens and Mycobacterium tuberculosis isolated from healthcare facilities in the Western Cape: an in-vitro study". J. Hosp. Infect. 68 (1): 45. doi:10.1016/j.jhin.2007.10.009. PMID 18069086.
{{cite journal}}: CS1 maint: multiple names: authors list (link) - ^ Gant VA, Wren MW, Rollins MS, Jeanes A, Hickok SS, Hall TJ (2007). "Three novel highly charged copper-based biocides: safety and efficacy against healthcare-associated organisms". J. Antimicrob. Chemother. 60 (2): 294. doi:10.1093/jac/dkm201. PMID 17567632.
{{cite journal}}: CS1 maint: multiple names: authors list (link) - ^ Noyce JO, Michels H, Keevil CW (2007). "Inactivation of influenza A virus on copper versus stainless steel surfaces". Appl. Environ. Microbiol. 73 (8): 2748. doi:10.1128/AEM.01139-06. PMC 1855605. PMID 17259354.
{{cite journal}}: CS1 maint: multiple names: authors list (link) - ^ "EPA registers copper-containing alloy products". US Environmental Protection Agency. Retrieved 2009-06-06.
- ^ "Center for Decease Control and Prevention" (PDF). Retrieved 2009-06-06.
- ^ "Merck Manuals – Online Medical Library: Copper". Merck. November 2005. Retrieved 2008-07-19.
- ^ "Pesticide Information Profile for Copper Sulfate". Cornell University. Retrieved 2008-07-10.
Notes
 |
 |
 |
 |
| in pure water, or acidic or alkali conditions. Copper in neutral water is more noble than hydrogen. | in water containing sulfide | in 10 M ammonia solution | in a chloride solution |
Further reading
- Massaro, Edward J., ed. (2002). Handbook of Copper Pharmacology and Toxicology. Humana Press. ISBN 0-89603-943-9.
- "Copper: Technology & Competitiveness (Summary) Chapter 6: Copper Production Technology" (PDF). Office of Technology Assessment. 2005.
- Current Medicinal Chemistry, Volume 12, Number 10, May 2005, pp. 1161–1208(48) Metals, Toxicity and Oxidative Stress
- William D. Callister (2003). Materials Science and Engineering: an Introduction, 6th Ed. Table 6.1, p. 137: Wiley, New York. ISBN 0471736961.
{{cite book}}: CS1 maint: location (link) - Material: Copper (Cu), bulk, MEMS and Nanotechnology Clearinghouse.
- Kim BE, Nevitt T, Thiele DJ (2008). "Mechanisms for copper acquisition, distribution and regulation". Nat. Chem. Biol. 4 (3): 176. doi:10.1038/nchembio.72. PMID 18277979.
{{cite journal}}: CS1 maint: multiple names: authors list (link) - Copper transport disorders: an Instant insight from the Royal Society of Chemistry
External links
- National Pollutant Inventory – Copper and compounds fact sheet
- Copper Resource Page. Includes 12 PDF files detailing the material properties of various kinds of copper, as well as various guides and tools for the copper industry.
- The Copper Development Association has an extensive site of properties and uses of copper; it also maintains a web site dedicated to brass, a copper alloy.
- The Third Millennium Online page on Copper
- Price history of copper, according to the IMF


