 | |
| Names | |
|---|---|
| Systematic IUPAC name
3-Butenenitrile | |
| Other names
Pyruvonitrile; Propanenitrile, 2-oxo-; α-Oxopropionitrile; Oxopropionitrile; Oxypropionitrile; Pyruvic acid nitrile; 2-Oxopropionitrile; CH3C(O)CN; 2-Oxopropanenitrile; 2-Oxopropiononitrile
| |
| Identifiers | |
3D model (JSmol)
|
|
| 605352 | |
| ChemSpider | |
| ECHA InfoCard | 100.003.366 |
| EC Number |
|
PubChem CID
|
|
CompTox Dashboard (EPA)
|
|
| |
| |
| Properties | |
| C4H5N | |
| Molar mass | 67.090 |
| Appearance | Clear light yellow liquid |
| Density | 0.834 g/cm3 |
| Melting point | -87 celcius |
| Boiling point | 116 - 121 °C (241 - 250 °F) |
| Water soluble | |
| Solubility | Immiscible in ethanol and ethyl ether |
| Hazards | |
| Occupational safety and health (OHS/OSH): | |
Main hazards
|
Flammable, Poison, irritates skin and eyes |
Ingestion hazards
|
Toxic if swallowed. |
Inhalation hazards
|
May be fatal if inhaled. Causes respiratory tract irritation. |
Eye hazards
|
Causes eye irritation. |
Skin hazards
|
Causes skin irritation. |
| GHS labelling: | |
 
| |
| Danger | |
| H226 H301 H315 H312 H319 H311 | |
| P280 P261 P305+P351+P338 P301+P310 P311 | |
| NFPA 704 (fire diamond) | |
| Flash point | 24 °C (75 °F) |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa).
| |
Allyl Cyanide
Allyl cyanide is a chemical compound. It is a nitrile, as it contains a carbon-nitrogen triple bond. Allyl cyanide has a scent similar to that of an onion. [1] At room temperature it exists as a clear, yellow liquid.[2] It is soluble in water, but it is immiscible in ethanol and ethyl ether. It has an index of refraction (n) of 1.4060.[3] Allyl cyanide is used in polymerization as a cross-linking agent. [4]
Synthesis
In an organic chemistry laboratory, allyl cyanide can be synthesized. This involves using a round bottom flask, a condenser, and a stir bar to perform a reflux. An example of reactants that can be used is allyl bromide and cuprous cyanide. The initial reaction is vigorous, and the heat source from the reflux must be replaced with an ice bath. This is because the reaction is so vigorous that some of the product could be lost via the condenser. After the vigorous reaction ceases, the reaction flask is refluxed again until there is no more allyl bromide to reflux. Distillation should be performed in order to purify the allyl cyanide. This has been the method proven to produce the highest yields. The same procedure can also be performed using potassium cyanide with allyl chloride, allyl bromide, or allyl iodide. Two other combinations of reactions are allyl chloride with cuprous cyanide or allyl alcohol, cuprous cyanide, and hydrochloric acid. [5]
Reactions
Hydrocyanation is a common reaction that occurs with alkyl nitriles. Alkyl nitriles are intermediates in the synthesis of amides, amines, carboxylic acids, and esters. Nickel-catalyzed hydrocyanation creates adiponitrile, as shown in the image below. Adiponitrile can be used to make hexamethylenediamine, which is used to produce Nylon. There are three steps in this process. The first is hydrocyanation of a butadiene to create a butenenitrile, such as allyl cyanide, and a pentenenitrile. The second is an isomerization, and the third is a second hydrocyanation. [6]
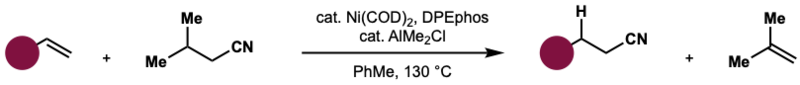
Natural Formation/Occurrences
One way that allyl cyanide is formed is through the breakdown of glucosinolates. Glucosinolates are found in plants such as kale and fresh cabbage. Glucosinolates are harmless when they are inside the plant, but when they are digested they decompose into more toxic components such as allyl cyanide. [7]
Allyl cyanide is also a component of mustard oil. [8]
Toxicology
Experiments have been done on rats to determine the effects of allyl cyanide (also known as 3-butenenitrile), along with cis-2-butenenitrile and trans-2-butenenitrile, on auditory and visual functioning. When the rats took the three unsaturated nitriles for 12 weeks, they became deaf and had a decline in vision. [9]
Allyl cyanide is formed from the breakdown of glucosinolates when ruminants such as sheep eat plants containing glucosinolates. However, sheep are far more tolerant to the toxic effects of allyl cyanide than rats. Studies suggest that this detoxification is due to rumen activity. [10]
Hazards
If swallowed, allyl cyanide will irritate the digestive track. It is also known to cause skin, respiratory tract, and eye irritation. It can cause dizziness, suffocation, impairment of cellular respiration, loss of appetite, headaches, weakness, nausea, and dizziness. Some of these effects only occur when allyl cyanide is metabolized and turned into cyanide. The compound is stable at normal temperatures and pressures; however it is highly flammable. This means that it should be stored away from and kept away from heat, sparks, and flames. Water should not be used to put out any fires caused by allyl cyanide. Water could spread the fire, but dry chemical, carbon dioxide, and alcohol-resistant foam are good options to extinguish a small fire. The vapor is also typically denser than air. This causes the vapor to sink to the ground and spread to confined areas.[11]
References
- ^ http://encyclopedia2.thefreedictionary.com/allyl+cyanide
- ^ http://www.sigmaaldrich.com/MSDS/MSDS/DisplayMSDSPage.do?country=US&language=en&productNumber=122793&brand=ALDRICH&PageToGoToURL=http3A%2F2Fwww.sigmaaldrich.com%2Fcatalog%2Fsearch%3Finterface%3DAll%26term%3DAllyl%2Bcyanide%26lang%3Den%26region%3DUS%26focus%3Dproduct%26N%3D0%2B220003048%2B219853269%2B219853286%26mode%3Dmatch%2520partialmax
- ^ Lide, David R., W. M. Haynes, and Thomas J. Bruno, eds. CRC Handbook of Chemistry and Physics. 93rd ed. Boca Raton, FL: CRC, 2012. Web. 17 Oct. 2012.
- ^ http://encyclopedia2.thefreedictionary.com/allyl+cyanide
- ^ http://www.orgsyn.org/orgsyn/orgsyn/prepcontent.asp?print=1&showprint=1&prep=cv1p0046
- ^ http://en.wikipedia.org/wiki/hydrocyanation
- ^ Duncan, A. J. and Milne, J. A. (1992), Rumen microbial degradation of allyl cyanide as a possible explanation for the tolerance of sheep to brassica-derived glucosinolates. J. Sci. Food Agric., 58: 15–19.
- ^ www.hort.purdue.edu/newcrop/med-aro/factsheets/mustard.html
- ^ Langlais, Cristina, Masarin Ban, Brigitte Marignac, and Francois Gagnaire. "The Ototoxic Effects Induced in Rats by Treatment for 12 Weeks with 2-butenenitrile, 3-butenenitrile and Cis-2-pentenenitrile." Pharmacology and Toxicology 88.3 (2001): 126-2. Biological Abstracts® - Web of Knowledge - Thomson Reuters. Web. 16 Oct. 2012.
- ^ Duncan, A. J. and Milne, J. A. (1992), Rumen microbial degradation of allyl cyanide as a possible explanation for the tolerance of sheep to brassica-derived glucosinolates. J. Sci. Food Agric., 58: 15–19.
- ^ http://fscimage.fishersci.com/msds/09962.htm
- http://www.sigmaaldrich.com/MSDS/MSDS/DisplayMSDSPage.do?country=US&language=en&productNumber=122793&brand=ALDRICH&PageToGoToURL=http%3A%2F%2Fwww.sigmaaldrich.com%2Fcatalog%2Fsearch%3Finterface%3DAll%26term%3DAllyl%2Bcyanide%26lang%3Den%26region%3DUS%26focus%3Dproduct%26N%3D0%2B220003048%2B219853269%2B219853286%26mode%3Dmatch%2520partialmax
- http://pubchem.ncbi.nlm.nih.gov/summary/summary.cgi?cid=8009
- http://www.chemspider.com/Chemical-Structure.13876176.html?rid=3ce850b8-d5dd-4133-af0b-4bc56fc442fa
