 | |
| Clinical data | |
|---|---|
| AHFS/Drugs.com | International Drug Names |
| Routes of administration | Intraperitoneal |
| ATC code | |
| Legal status | |
| Legal status |
|
| Pharmacokinetic data | |
| Bioavailability | 40% in 12 hours |
| Metabolism | Alpha-amylase |
| Excretion | Renal |
| Identifiers | |
| CAS Number | |
| PubChem SID | |
| DrugBank | |
| ChemSpider |
|
| UNII | |
| KEGG | |
| ChEMBL | |
| CompTox Dashboard (EPA) | |
| Chemical and physical data | |
| Formula | (C6H10O5)n |
| Molar mass | 13–19 kg/mol |
| | |
Icodextrin (INN, USAN) is a colloid osmotic agent, derived from maltodextrin,[1] used in form of an aqueous solution for peritoneal dialysis under the trade name Extraneal,[2] and after gynecological laparoscopic surgery for the reduction of post-surgical adhesions (fibrous bands that form between tissues and organs) under the trade name Adept.[3]
Chemistry[edit]
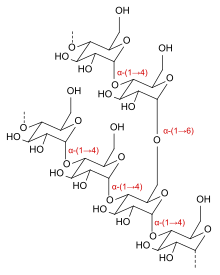
Icodextrin is a starch-derived, branched, water-soluble glucose polymer linked by α-(1→4) and less than 10% α-(1→6) glycosidic bonds, making it a type of dextrin. Its weight-average molecular weight is between 13,000 and 19,000 daltons and its number-average molecular weight between 5,000 and 6,500 daltons. The substance is a white to off-white solid, and the solution is clear and colourless to pale yellow.[3]
Mechanism of action[edit]
The osmotic activity of icodextrin keeps the solution inside the peritoneum for three to four days, separating tissues and thus reducing adhesion between them when fibrin is formed after a surgery. In other words, the tissues are kept from gluing together.[3]

When used for peritoneal dialysis, the icodextrin solution absorbs waste products from the blood, and is removed from the peritoneum after a few hours together with the waste.[4]
Pharmacokinetics[edit]
Icodextrin is not significantly metabolised inside the peritoneum. Instead, it is absorbed slowly (40% after 12 hours) into the bloodstream via the lymph vessels. There it is broken down into oligosaccharides by the enzyme alpha-amylase. In patients with intact kidney function, both icodextrin and its fragments are excreted via the kidney by glomerular filtration.[2][3]
Contraindications[edit]
Icodextrin is contraindicated in patients with cornstarch allergy, maltose or isomaltose intolerance, glycogen storage disease, or severe lactic acidosis.[5]
Adverse effects[edit]
Adverse effects include peritonitis, respiratory infection, hypertension (high blood pressure), rashes, and headache. Of these side effects, only hypertension and rashes occurred significantly more often than under glucose solution; the other events seem to be related to peritoneal dialysis in general.[5]
Interactions[edit]
Icodextrin can mimic increased blood glucose levels, depending on the used testing system. Specifically, glucose dehydrogenase pyrroloquinolinequinone (GDH-PQQ) or glucose-dye-oxidoreductase (GDO) based tests can erroneously show high blood glucose in patients that have been treated with icodextrin.[5]
References[edit]
- ^ American College of Physicians--American Society of Internal Medicine (2005). Clinical evidence, Issue 14. BMJ Pub. Group. p. 1046.
- ^ a b "Extraneal". RxList.com.
- ^ a b c d "Adept (4% Icodextrin) Adhesion Reduction Solution" (PDF). FDA.
- ^ Frampton JE, Plosker GL (2003). "Icodextrin: a review of its use in peritoneal dialysis". Drugs. 63 (19): 2079–105. doi:10.2165/00003495-200363190-00011. PMID 12962523.
- ^ a b c "Extraneal". Drugs.com.