 | |
 | |
| Clinical data | |
|---|---|
| Trade names | Noristerat, others |
| Other names | NETE; NET-EN; Norethindrone enanthate; SH-393; 17α-Ethynyl-19-nortestosterone 17β-enanthate; 17α-Ethynylestra-4-en-17β-ol-3-one 17β-enanthate |
| AHFS/Drugs.com | International Drug Names |
| Routes of administration | Intramuscular injection |
| Drug class | Progestogen; Progestin; Progestogen ester |
| ATC code | |
| Legal status | |
| Legal status |
|
| Identifiers | |
| |
| CAS Number | |
| PubChem CID | |
| DrugBank | |
| ChemSpider | |
| UNII | |
| KEGG | |
| ChEBI | |
| ChEMBL | |
| CompTox Dashboard (EPA) | |
| ECHA InfoCard | 100.021.207 |
| Chemical and physical data | |
| Formula | C27H38O3 |
| Molar mass | 410.598 g·mol−1 |
| 3D model (JSmol) | |
| |
| |
| (verify) | |
Norethisterone enanthate (NETE), also known as norethindrone enanthate, is a form of hormonal birth control which is used to prevent pregnancy in women.[1][2][3] It is used both as a form of progestogen-only injectable birth control and in combined injectable birth control formulations. It may be used following childbirth, miscarriage, or abortion.[1] The failure rate per year in preventing pregnancy for the progestogen-only formulation is 2 per 100 women.[4] Each dose of this form lasts two months with only up to two doses typically recommended.[5][1]
Side effects include breast pain, headaches, depression, irregular menstrual periods, and pain at the site of injection.[5] Use in those with liver disease is not recommended as is use during pregnancy due to risk of birth defects.[1] Use appears to be okay during breastfeeding.[1] It does not protect against sexually transmitted infections.[1] NETE is an ester and prodrug of norethisterone,[6] through which it works.[1] It works as a method of birth control by stopping ovulation.[1]
Norethisterone was patented in 1951 and NETE came into medical use in 1957.[7][8] It is on the World Health Organization's List of Essential Medicines.[9] It has been approved by itself in more than 60 countries including the United Kingdom and some in Europe, Central America, and Africa, and in combination with estradiol valerate in at least 36 countries mainly in Latin America.[4][10][11][12] It is not available in the United States.[10]
Medical uses[edit]
NETE is used on its own as a long-lasting progestogen-only injectable contraceptive in women.[1][5] It is administered via intramuscular injection once every two months.[1][5]
Contraindications[edit]
Side effects[edit]
Side effects of NETE may include breast pain, headaches, depression, irregular menstrual periods, and pain at the site of injection.[5] It can cause birth defects in the fetus if used during pregnancy.[1]
Overdose[edit]
Interactions[edit]
Pharmacology[edit]

Pharmacodynamics[edit]
NETE is a prodrug of norethisterone in the body.[13] Upon reaching circulation, it is rapidly converted into norethisterone by esterases. Hence, as a prodrug of norethisterone, NETE has essentially the same effects as norethisterone, acting as a potent progestogen with additional weak androgenic and estrogenic activity (the latter via its metabolite ethinylestradiol).[14] NETA has some progestogenic activity of its own, but it is unclear if NETE does similarly.[13]
NETE is of about 38% higher molecular weight than norethisterone due to the presence of its C17β enanthate ester.[2]
| Compound | Typea | PR | AR | ER | GR | MR | SHBG | CBG |
|---|---|---|---|---|---|---|---|---|
| Norethisterone | – | 67–75 | 15 | 0 | 0–1 | 0–3 | 16 | 0 |
| 5α-Dihydronorethisterone | Metabolite | 25 | 27 | 0 | 0 | ? | ? | ? |
| 3α,5α-Tetrahydronorethisterone | Metabolite | 1 | 0 | 0–1 | 0 | ? | ? | ? |
| 3α,5β-Tetrahydronorethisterone | Metabolite | ? | 0 | 0 | ? | ? | ? | ? |
| 3β,5α-Tetrahydronorethisterone | Metabolite | 1 | 0 | 0–8 | 0 | ? | ? | ? |
| Ethinylestradiol | Metabolite | 15–25 | 1–3 | 112 | 1–3 | 0 | 0.18 | 0 |
| Norethisterone acetate | Prodrug | 20 | 5 | 1 | 0 | 0 | ? | ? |
| Norethisterone enanthate | Prodrug | ? | ? | ? | ? | ? | ? | ? |
| Noretynodrel | Prodrug | 6 | 0 | 2 | 0 | 0 | 0 | 0 |
| Etynodiol | Prodrug | 1 | 0 | 11–18 | 0 | ? | ? | ? |
| Etynodiol diacetate | Prodrug | 1 | 0 | 0 | 0 | 0 | ? | ? |
| Lynestrenol | Prodrug | 1 | 1 | 3 | 0 | 0 | ? | ? |
| Notes: Values are percentages (%). Reference ligands (100%) were promegestone for the PR, metribolone for the AR, estradiol for the ER, dexamethasone for the GR, aldosterone for the MR, dihydrotestosterone for SHBG, and cortisol for CBG. Footnotes: a = Active or inactive metabolite, prodrug, or neither of norethisterone. Sources: See template. | ||||||||
| Compound | Form | Dose for specific uses (mg)[c] | DOA[d] | |||
|---|---|---|---|---|---|---|
| TFD[e] | POICD[f] | CICD[g] | ||||
| Algestone acetophenide | Oil soln. | - | – | 75–150 | 14–32 d | |
| Gestonorone caproate | Oil soln. | 25–50 | – | – | 8–13 d | |
| Hydroxyprogest. acetate[h] | Aq. susp. | 350 | – | – | 9–16 d | |
| Hydroxyprogest. caproate | Oil soln. | 250–500[i] | – | 250–500 | 5–21 d | |
| Medroxyprog. acetate | Aq. susp. | 50–100 | 150 | 25 | 14–50+ d | |
| Megestrol acetate | Aq. susp. | - | – | 25 | >14 d | |
| Norethisterone enanthate | Oil soln. | 100–200 | 200 | 50 | 11–52 d | |
| Progesterone | Oil soln. | 200[i] | – | – | 2–6 d | |
| Aq. soln. | ? | – | – | 1–2 d | ||
| Aq. susp. | 50–200 | – | – | 7–14 d | ||
|
Notes and sources:
| ||||||

A single intramuscular injection of estradiol valerate/norethisterone enanthate (5 mg/50 mg) (Mesigyna) has been found to strongly suppress testosterone levels in men.[34] Levels of testosterone decreased from ~503 ng/dL at baseline to ~30 ng/dL at the lowest point (–94%).[34]
Pharmacokinetics[edit]

A single intramuscular injection of 50 to 200 mg NETE in oil solution has been found to have a duration of action of 11 to 52 days in terms of clinical biological effect in the uterus and on body temperature in women.[36]
Similarly to oral norethisterone and norethisterone acetate, intramuscular NETE has been found to form ethinylestradiol as an active metabolite.[35] With a single intramuscular injection of 200 mg NETE in premenopausal women, the mean maximum concentration of ethinylestradiol was 32% of that of a combined oral contraceptive containing 30 μg ethinylestradiol, the maximum equivalent oral dose of ethinylestradiol observed in the first few days of exposure was 20.3 μg/day, and the mean equivalent oral dose of ethinylestradiol over 8 weeks was 4.41 μg/day.[35] As such, the exposure to ethinylestradiol was described as markedly lower than that of an oral contraceptive containing 30 μg ethinylestradiol.[35] The estimated conversion rate of NETE into ethinylestradiol was 0.1%, which was much lower than that observed for oral norethisterone and norethisterone enanthate (0.2–1.0%), likely due to the lack of the first pass through the liver with parenteral administration.[35] In accordance with the low levels of ethinylestradiol produced, no increase rates of thromboembolism or hepatic adenoma have been observed in post-authorization data of intramuscular NETE, and the medication does not resemble combined oral contraceptives containing ethinylestradiol in its safety profile.[35]
Chemistry[edit]
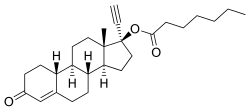
NETE, also known as norethinyltestosterone enanthate, as well as 17α-ethynyl-19-nortestosterone 17β-enanthate or 17α-ethynylestr-4-en-17β-ol-3-one 17β-enanthate, is a progestin, or synthetic progestogen, of the 19-nortestosterone group, and a synthetic estrane steroid.[2][37] It is the C17β enanthate ester of norethisterone.[2][37] NETE is a derivative of testosterone with an ethynyl group at the C17α position, the methyl group at the C19 position removed, and an enanthate ester attached at the C17β position.[2][37] In addition to testosterone, it is a combined derivative of nandrolone (19-nortestosterone) and ethisterone (17α-ethynyltestosterone).[2][37] Esters related to NETE include norethisterone acetate and levonorgestrel butanoate.[2][37]
History[edit]
NETE was introduced by Schering as Noristerat in 1957.[8] It was the second long-acting progestogen to be used clinically, after hydroxyprogesterone caproate.[38] The medication was the first progestogen-only injectable contraceptive, preceding medroxyprogesterone acetate (Depo-Provera).[8]
Society and culture[edit]
Generic names[edit]
Norethisterone enantate is the generic name of the drug and its INNM and BANM.[2][37][39][40][41] It is also spelled as norethisterone enanthate and is also known as norethindrone enanthate (the USAN of norethisterone being norethindrone).[2][37][39][40][41] NETE is known by its former developmental code name SH-393 as well.[2][37][39][40][41]
Brand names[edit]
NETE has been marketed alone as a progestogen-only injectable contraceptive under the brand names Depocon, Doryxas, NET-EN, Noristat, Noristerat, Norigest, and Nur-Isterate, and in combination with estradiol valerate as a combined injectable contraceptive under the brand names Chinese Injectable No. 3, Efectimes, Ginediol, Mesigyna, Mesilar, Meslart, Mesocept, Mesygest, Nofertyl, Nofertyl Lafrancol, Noregyna, Norestrin, Norifam, Norigynon, Nostidyn, Sexseg, and Solouna.[37][40][41][42]
| Composition | Dose | Brand names | Use |
|---|---|---|---|
| NET only | Low (e.g., 0.35 mg) | Multiple[a] | Progestogen-only oral contraceptive |
| NET or NETA only | High (e.g., 5 mg, 10 mg) | Multiple[b] | Gynecological disorders and other uses |
| NETE only | Injection (e.g., 200 mg) | Multiple[c] | Progestogen-only injectable contraceptive |
| NET or NETA with ethinylestradiol | Low (e.g., 0.4 mg, 0.5 mg, 0.75 mg, 1 mg, 1.5 mg) | Multiple[d] | Combined oral contraceptive |
| NET with mestranol | Low (e.g., 1 mg, 2 mg) | Multiple[e] | Combined oral contraceptive |
| NETA with estradiol | Low (e.g., 0.1 mg, 0.5 mg) | Multiple[f] | Combined menopausal hormone therapy |
| NETE with estradiol valerate | Injection (e.g., 50 mg) | Multiple[g] | Combined injectable contraceptive |
| Abbreviations: NET = Norethisterone. NETA = Norethisterone acetate. NETE = Norethisterone enanthate. Sources: [43][44] [37][45] Notes:
| |||
Availability[edit]
NETE has been approved for use alone as a progestogen-only injectable contraceptive in more than 60 countries throughout the world including in Europe, Latin America, Asia, and Africa.[4][10][11] Specific countries in which NETE as a standalone medication is or has been available include Bangladesh, France, Germany, India, Italy, Malaysia, Mexico, the Philippines, Singapore, South Africa, Thailand, and the United Kingdom.[37][40][41][42]
NETE has been approved for use in combination with estradiol valerate as a combined injectable contraceptive in at least 36 countries, mostly in Latin America but also in Africa.[11][12] It is or has been available in combination with estradiol valerate in Argentina, the Bahamas, Barbados, Bolivia, Brazil, Chile, Colombia, Costa Rica, the Dominican Republic, Ecuador, Egypt, El Salvador, Ghana, Grenada, Guatemala, Guyana, Haiti, Honduras, Jamaica, Kenya, Mexico, Nicaragua, Panama, Paraguay, Peru, St. Lucia, Turkey, Uruguay, Venezuela, and Zimbabwe.[40][41][42][14]
NETE is not available in any form in the United States.[10]
Research[edit]
NETE was studied by Schering for use as a progestogen-only injectable contraceptive at a dose of 25 mg once a month but produced poor cycle control with this regimen and was not marketed.[46]
NETE has been studied for use as a potential male hormonal contraceptive in combination with testosterone in men.[47]
See also[edit]
References[edit]
- ^ a b c d e f g h i j k "Noristerat 200mg, solution for intramuscular injection - Summary of Product Characteristics (SPC) - (eMC)". www.medicines.org.uk. Archived from the original on 31 December 2016. Retrieved 31 December 2016.
- ^ a b c d e f g h i j Elks J (14 November 2014). The Dictionary of Drugs: Chemical Data: Chemical Data, Structures and Bibliographies. Springer. pp. 886–. ISBN 978-1-4757-2085-3. Archived from the original on 5 November 2017.
- ^ Index Nominum 2000: International Drug Directory. Taylor & Francis US. 2000. p. 750. ISBN 978-3-88763-075-1. Archived from the original on 28 May 2013. Retrieved 30 May 2012.
- ^ a b c Committee on Contraceptive Development (U.S.) (1 January 1990). Mastroianni L, Donaldson PJ, Kane TT (eds.). Developing New Contraceptives: Obstacles and Opportunities. National Academies. pp. 38–. ISBN 9780309041478. NAP:14119.
- ^ a b c d e World Health Organization (2009). Stuart MC, Kouimtzi M, Hill SR (eds.). WHO Model Formulary 2008. World Health Organization. p. 370. hdl:10665/44053. ISBN 9789241547659.
- ^ Wu L, Janagam DR, Mandrell TD, Johnson JR, Lowe TL (2015). "Long-acting injectable hormonal dosage forms for contraception". Pharmaceutical Research. 32 (7): 2180–91. doi:10.1007/s11095-015-1686-2. PMID 25899076. S2CID 12856674.
- ^ Fischer J, Ganellin CR (2006). Analogue-based Drug Discovery. John Wiley & Sons. p. 478. ISBN 9783527607495. Archived from the original on 2016-12-20.
- ^ a b c Bullough VL (2001). Encyclopedia of Birth Control. ABC-CLIO. pp. 145–. ISBN 978-1-57607-181-6. Archived from the original on 2017-11-05.
- ^ World Health Organization (2019). World Health Organization model list of essential medicines: 21st list 2019. Geneva: World Health Organization. hdl:10665/325771. WHO/MVP/EMP/IAU/2019.06. License: CC BY-NC-SA 3.0 IGO.
- ^ a b c d Whitaker A, Gilliam M (27 June 2014). Contraception for Adolescent and Young Adult Women. Springer. p. 96. ISBN 978-1-4614-6579-9. Archived from the original on 5 November 2017.
- ^ a b c Bagade O, Pawar V, Patel R, Patel B, Awasarkar V, Diwate S (2014). "Increasing use of long-acting reversible contraception: safe, reliable, and cost-effective birth control" (PDF). World J Pharm Pharm Sci. 3 (10): 364–392. ISSN 2278-4357. Archived from the original (PDF) on 2017-08-10. Retrieved 2018-08-02.
- ^ a b Newton JR, D'arcangues C, Hall PE (1994). "A review of "once-a-month" combined injectable contraceptives". J Obstet Gynaecol (Lahore). 4 (Suppl 1): S1–34. doi:10.3109/01443619409027641. PMID 12290848.
- ^ a b Hapgood JP, Koubovec D, Louw A, Africander D (November 2004). "Not all progestins are the same: implications for usage". Trends Pharmacol. Sci. 25 (11): 554–7. doi:10.1016/j.tips.2004.09.005. PMID 15491776.
- ^ a b IARC Working Group on the Evaluation of Carcinogenic Risks to Humans; World Health Organization; International Agency for Research on Cancer (2007). Combined Estrogen-progestogen Contraceptives and Combined Estrogen-progestogen Menopausal Therapy. World Health Organization. pp. 417, 432. ISBN 978-92-832-1291-1. Archived from the original on 2017-11-05.
Norethisterone and its acetate and enanthate esters are progestogens that have weak estrogenic and androgenic properties.
- ^ Knörr K, Beller FK, Lauritzen C (17 April 2013). Lehrbuch der Gynäkologie. Springer-Verlag. pp. 214–. ISBN 978-3-662-00942-0.
- ^ Knörr K, Knörr-Gärtner H, Beller FK, Lauritzen C (8 March 2013). Geburtshilfe und Gynäkologie: Physiologie und Pathologie der Reproduktion. Springer-Verlag. pp. 583–. ISBN 978-3-642-95583-9.
- ^ Labhart A (6 December 2012). Clinical Endocrinology: Theory and Practice. Springer Science & Business Media. pp. 554–. ISBN 978-3-642-96158-8.
- ^ Horský J, Presl J (1981). "Hormonal Treatment of Disorders of the Menstrual Cycle". In Horsky J, Presl K (eds.). Ovarian Function and its Disorders: Diagnosis and Therapy. Springer Science & Business Media. pp. 309–332. doi:10.1007/978-94-009-8195-9_11. ISBN 978-94-009-8195-9.
- ^ Ufer J (1969). The Principles and Practice of Hormone Therapy in Gynaecology and Obstetrics. de Gruyter. p. 49. ISBN 9783110006148.
17α-Hydroxyprogesterone caproate is a depot progestogen which is entirely free of side actions. The dose required to induce secretory changes in primed endometrium is about 250 mg. per menstrual cycle.
- ^ Pschyrembel W (1968). Praktische Gynäkologie: für Studierende und Ärzte. Walter de Gruyter. pp. 598, 601. ISBN 978-3-11-150424-7.
- ^ Ferin J (September 1972). "Effects, Duration of Action and Metabolism in Man". In Tausk M (ed.). Pharmacology of the Endocrine System and Related Drugs: Progesterone, Progestational Drugs and Antifertility Agents. Vol. II. Pergamon Press. pp. 13–24. ISBN 978-0080168128. OCLC 278011135.
- ^ Henzl MR, Edwards JA (10 November 1999). "Pharmacology of Progestins: 17α-Hydroxyprogesterone Derivatives and Progestins of the First and Second Generation". In Sitruk-Ware R, Mishell DR (eds.). Progestins and Antiprogestins in Clinical Practice. Taylor & Francis. pp. 101–132. ISBN 978-0-8247-8291-7.
- ^ Brotherton J (1976). Sex Hormone Pharmacology. Academic Press. p. 114. ISBN 978-0-12-137250-7.
- ^ Sang GW (April 1994). "Pharmacodynamic effects of once-a-month combined injectable contraceptives". Contraception. 49 (4): 361–385. doi:10.1016/0010-7824(94)90033-7. PMID 8013220.
- ^ Toppozada MK (April 1994). "Existing once-a-month combined injectable contraceptives". Contraception. 49 (4): 293–301. doi:10.1016/0010-7824(94)90029-9. PMID 8013216.
- ^ Goebelsmann U (1986). "Pharmacokinetics of Contraceptive Steroids in Humans". In Gregoire AT, Blye RP (eds.). Contraceptive Steroids: Pharmacology and Safety. Springer Science & Business Media. pp. 67–111. doi:10.1007/978-1-4613-2241-2_4. ISBN 978-1-4613-2241-2.
- ^ Becker H, Düsterberg B, Klosterhalfen H (1980). "[Bioavailability of cyproterone acetate after oral and intramuscular application in men (author's transl)]" [Bioavailability of Cyproterone Acetate after Oral and Intramuscular Application in Men]. Urologia Internationalis. 35 (6): 381–385. doi:10.1159/000280353. PMID 6452729.
- ^ Moltz L, Haase F, Schwartz U, Hammerstein J (May 1983). "[Treatment of virilized women with intramuscular administration of cyproterone acetate]" [Efficacy of Intra muscularly Applied Cyproterone Acetate in Hyperandrogenism]. Geburtshilfe und Frauenheilkunde. 43 (5): 281–287. doi:10.1055/s-2008-1036893. PMID 6223851.
- ^ Wright JC, Burgess DJ (29 January 2012). Long Acting Injections and Implants. Springer Science & Business Media. pp. 114–. ISBN 978-1-4614-0554-2.
- ^ Chu YH, Li Q, Zhao ZF (April 1986). "Pharmacokinetics of megestrol acetate in women receiving IM injection of estradiol-megestrol long-acting injectable contraceptive". The Chinese Journal of Clinical Pharmacology.
The results showed that after injection the concentration of plasma MA increased rapidly. The meantime of peak plasma MA level was 3rd day, there was a linear relationship between log of plasma MA concentration and time (day) after administration in all subjects, elimination phase half-life t1/2β = 14.35 ± 9.1 days.
- ^ Runnebaum BC, Rabe T, Kiesel L (6 December 2012). Female Contraception: Update and Trends. Springer Science & Business Media. pp. 429–. ISBN 978-3-642-73790-9.
- ^ Artini PG, Genazzani AR, Petraglia F (11 December 2001). Advances in Gynecological Endocrinology. CRC Press. pp. 105–. ISBN 978-1-84214-071-0.
- ^ King TL, Brucker MC, Kriebs JM, Fahey JO (21 October 2013). Varney's Midwifery. Jones & Bartlett Publishers. pp. 495–. ISBN 978-1-284-02542-2.
- ^ a b c d del Cisne Valle Alvarez D (11 May 2011). Efecto de una Dosis de 50 mg de Enantato de Noretisterona y 5 mg de Valerato de Estradiol en los Niveles de Testosterona Total en Hombres Mexicanos Sanos [Effect of a Dose of 50 mg of Norethisterone Enanthate and 5 mg of Estradiol Valerate on Total Testosterone Levels in Healthy Mexican Men] (MSc). National Polytechnic Institute of Mexico.
- ^ a b c d e f Friedrich C, Berse M, Klein S, Rohde B, Höchel J (March 2018). "In Vivo Formation of Ethinylestradiol After Intramuscular Administration of Norethisterone Enantate". J Clin Pharmacol. 58 (6): 781–789. doi:10.1002/jcph.1079. PMID 29522253. S2CID 3813229.
- ^ Ferin J (September 1972). "Effects, Duration of Action and Metabolism in Man". In Tausk M (ed.). Pharmacology of the Endocrine System and Related Drugs: Progesterone, Progestational Drugs and Antifertility Agents. Vol. II. Pergamon Press. pp. 13–24. ISBN 978-0080168128. OCLC 278011135.
- ^ a b c d e f g h i j k Index Nominum 2000: International Drug Directory. Taylor & Francis. January 2000. pp. 749–. ISBN 978-3-88763-075-1.
- ^ Boschann HW (July 1958). "Observations of the role of progestational agents in human gynecologic disorders and pregnancy complications". Ann. N. Y. Acad. Sci. 71 (5): 727–52. Bibcode:1958NYASA..71..727B. doi:10.1111/j.1749-6632.1958.tb46803.x. PMID 13583829.
- ^ a b c Morton IK, Hall JM (6 December 2012). Concise Dictionary of Pharmacological Agents: Properties and Synonyms. Springer Science & Business Media. pp. 201–. ISBN 978-94-011-4439-1.
- ^ a b c d e f "Norethisterone".
- ^ a b c d e f Sweetman SC, ed. (2009). "Sex hormones and their modulators". Martindale: The Complete Drug Reference (36th ed.). London: Pharmaceutical Press. p. 2082. ISBN 978-0-85369-840-1.
- ^ a b c "Micromedex Products: Please Login".
- ^ "Norethisterone". Drugs.com.
- ^ "Drugs@FDA: FDA Approved Drug Products". United States Food and Drug Administration. Archived from the original on 16 November 2016. Retrieved 27 November 2016.
- ^ IARC Working Group on the Evaluation of Carcinogenic Risks to Humans; International Agency for Research on Cancer (1 January 1999). Hormonal Contraception and Post-menopausal Hormonal Therapy (PDF). IARC. p. 65. ISBN 978-92-832-1272-0.
- ^ Toppozada M (June 1977). "The clinical use of monthly injectable contraceptive preparations". Obstet Gynecol Surv. 32 (6): 335–47. doi:10.1097/00006254-197706000-00001. PMID 865726.
- ^ Nieschlag E (2010). "Clinical trials in male hormonal contraception" (PDF). Contraception. 82 (5): 457–70. doi:10.1016/j.contraception.2010.03.020. PMID 20933120.
External links[edit]
- "Norethisterone Enanthate". Drug Information Portal. U.S. National Library of Medicine.