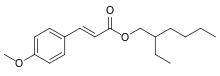
| |
| Names | |
|---|---|
| IUPAC name
(RS)-2-Ethylhexyl (2E)-3-(4-methoxyphenyl)prop-2-enoate
| |
| Other names
Ethylhexyl methoxycinnamate
Octinoxate Uvinul MC80 (E)-3-(4-methoxyphenyl) prop-2-enoic acid 2-ethylhexyl ester | |
| Identifiers | |
3D model (JSmol)
|
|
| ChEMBL | |
| ChemSpider | |
| ECHA InfoCard | 100.157.824 |
PubChem CID
|
|
| UNII | |
CompTox Dashboard (EPA)
|
|
| |
| |
| Properties | |
| C18H26O3 | |
| Molar mass | 290.403 g·mol−1 |
| Density | 1.01 g/cm3 |
| Melting point | −25 °C (−13 °F; 248 K) |
| Boiling point | 198 to 200 °C (388 to 392 °F; 471 to 473 K) |
| Pharmacology | |
| D02BA02 (WHO) | |
| Legal status |
|
| Hazards | |
| NFPA 704 (fire diamond) | |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa).
| |
Octyl methoxycinnamate or ethylhexyl methoxycinnamate (INCI) or octinoxate (USAN), trade names Eusolex 2292 and Uvinul MC80, is an organic compound that is an ingredient in some sunscreens and lip balms. It is an ester formed from methoxycinnamic acid and 2-ethylhexanol. It is a liquid that is insoluble in water.
It is primarily used in sunscreens and other cosmetics to absorb UV-B rays from the sun, protecting the skin from damage. It is also used to reduce the appearance of scars.
Uses[edit]
Octyl methoxycinnamate is the most common active ingredient in sunscreens for protection against UV-B rays.[2][3] It may be combined with oxybenzone and titanium oxide.[2]
Studies have evaluated the efficacy of octyl methoxycinnamate in preventing postoperative peritoneal adhesions and determined that octyl methoxycinnamate covering peritoneal surfaces decreases adhesion formation. This effect is more notable when octyl methoxycinnamate is applied before the induction of trauma.[4]
Chromophore groups, such as C=C, C=O, and O-N=O, have loosely held electrons that are excited by radiation. Hence, octyl methoxycinnamate is able to absorb radiation when the electron energy level is increased to an excited state.[5]
Properties[edit]
The UV spectra of octyl methoxycinnamate contains a maximum at 310 nm.[6]
Synthesis[edit]
Olefin metathesis has been widely studied. One of the synthesis pathways for octyl methoxycinnamate includes cross metathesis. The high efficiency of the nitro-Grela catalyst has been used in the cross metathesis of trans-anethole with 2-ethylhexyl acrylate to produce octyl methoxycinnamate (86% yield).[7]
Safety studies[edit]
One study performed in 2000 raised safety concerns about octyl methoxycinnamate by demonstrating toxicity to mouse cells at concentrations lower than typical levels in sunscreens.[8][9][medical citation needed] However, another study concluded that octyl methoxycinnamate and other sun screening agents do not penetrate the outer skin in sufficient concentration to cause any significant toxicity to the underlying human keratinocytes.[10]
Estrogenic and neurological effects were noted in laboratory animals at concentrations close to those experienced by sunscreen users[11][12] and were also shown in vitro.[citation needed] Octyl methoxycinnamate has been shown to be light sensitive with a decrease in UV absorption efficiency upon light exposure.[13] This degradation causes formation of the Z-octyl-p-methoxycinnamate from the E-octyl-p-methoxycinnamate. In contrast, the OMC does not show degradation when kept in darkness for extended periods of time.[citation needed]
A study carried out in 2017 by the Research Centre for Toxic Compounds in the Environment at Masaryk University, Czech Republic, indicates that octyl methoxycinnamate (EHMC) may damage human cell DNA. When exposed to sun rays, the spatial arrangement of its molecules changes and isomerisation takes place. While until now only unchanged EHMC has been researched, Massaryk University researchers focused on its isomers and found out that it has a significant genotoxic effect under lab conditions. It means that it may potentially damage human DNA and cause genome mutations which may lead to serious health risks.[14]
In swimming pools with hypochlorite in aqueous solution, octyl methoxycinnamate has been shown to produce chlorine-substituted intermediates. The chlorination intermediates of octyl methoxycinnamate demonstrated weak mutagenic effects on the Salmonella typhimurium TA 100 strain. The reactions depended on the pH, compound structures, and chlorine dose.[15]
Ecological damage[edit]
Concern about effects on coral reefs resulted in a bill in the state legislature of Hawaii to limit use of sunscreens containing octyl methoxycinnamate and oxybenzone.[16][17]
For the same reasons, the government of Palau signed a law in 2018 (becoming effective in 2020) that restricted the sale and use of sunscreen and skincare products that contain a list of ten different chemicals, including the UV filters octyl methoxycinnamate, oxybenzone and octocrylene, with fines of US$1,000 for retailers who violate the law and the power to confiscate such products from non-commercial users.[18]
Stereochemistry[edit]
Octinoxate contains a stereocenter and a double bond. It has the following stereoisomers[19][20] Therefore, octinoxate could consist of the following four stereoisomers:
| Enantiomers of Octinoxate | ||
|---|---|---|
| (R)-shape | (S)-shape | |
| (E)-shape | 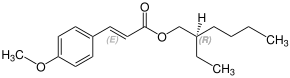
|

|
| (Z)-shape | 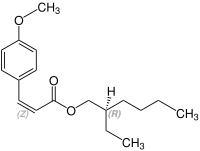
|
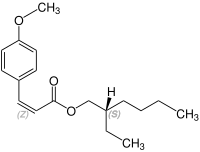
|
See also[edit]
- Amiloxate, a chemically related sunscreening agent
- Cinoxate, another cinnamic acid based sunscreen ingredient
- Avobenzone – UV-A protectant used in sunscreens
References[edit]
- ^ Merck Index, 11th Edition, 6687.
- ^ a b Serpone, Nick; Salinaro, Angela; Emeline, Alexei V.; Horikoshi, Satoshi; Hidaka, Hisao; Zhao, Jincai (2002). "An in vitro systematic spectroscopic examination of the photostabilities of a random set of commercial sunscreen lotions and their chemical UVB/UVA active agents". Photochemical & Photobiological Sciences. 1 (12): 970–81. doi:10.1039/B206338G. PMID 12661594. S2CID 27248506.
- ^ Ngan, Vanessa (2012). "Allergy to cinnamate". DermNet NZ. Retrieved 18 October 2021.
- ^ Aysan, Erhan; Bektas, Hasan; Kaygusuz, Arslan (December 2009). "Efficacy of octyl methoxycinnamate in preventing postoperative peritoneal adhesions: An experimental model". Journal of Obstetrics and Gynaecology Research. 35 (6): 1102–1108. doi:10.1111/j.1447-0756.2009.01077.x. PMID 20025636. S2CID 24582333.
- ^ PubChem. "Octinoxate". pubchem.ncbi.nlm.nih.gov. Retrieved 13 November 2021.
- ^ PubChem. "Octinoxate". pubchem.ncbi.nlm.nih.gov. Retrieved 13 November 2021.
- ^ Kaczanowska, Katarzyna; Trzaskowski, Bartosz; Peszczyńska, Aleksandra; Tracz, Andrzej; Gawin, Rafał; Olszewski, Tomasz K.; Skowerski, Krzysztof (2020). "Cross metathesis with acrylates: N-heterocyclic carbene (NHC)- versus cyclic alkyl amino carbene (CAAC)-based ruthenium catalysts, an unanticipated influence of the carbene type on efficiency and selectivity of the reaction". ChemCatChem. 12 (24): 6366–6374. doi:10.1002/cctc.202001268. ISSN 1867-3899. S2CID 225155345.
- ^ Sinister side of sunscreens, Rob Edwards, New Scientist, 7 October 2000
- ^ Butt, S.T.; Christensen, T. (2000). "Toxicity and Phototoxicity of Chemical Sun Filters". Radiation Protection Dosimetry. 91: 283–6. doi:10.1093/oxfordjournals.rpd.a033219. INIST 1532995.
- ^ Hayden, C.G.J.; Cross, S.E.; Anderson, C.; Saunders, N.A.; Roberts, M.S. (2005). "Sunscreen Penetration of Human Skin and Related Keratinocyte Toxicity after Topical Application". Skin Pharmacology and Physiology. 18 (4): 170–4. doi:10.1159/000085861. PMID 15908756. S2CID 7914504.
- ^ Petersen, Marta Axelstad (2011). Thyroid hormone disrupting chemicals and their influence on the developing rat brain (PhD Thesis). DTU Food, National Food Institute. ISBN 978-87-92158-94-9. OCLC 826386040.[page needed]
- ^ Axelstad, Marta; Boberg, Julie; Hougaard, Karin Sørig; Christiansen, Sofie; Jacobsen, Pernille Rosenskjold; Mandrup, Karen Riiber; Nellemann, Christine; Lund, Søren Peter; Hass, Ulla (2011). "Effects of pre- and postnatal exposure to the UV-filter Octyl Methoxycinnamate (OMC) on the reproductive, auditory and neurological development of rat offspring". Toxicology and Applied Pharmacology. 250 (3): 278–90. doi:10.1016/j.taap.2010.10.031. PMID 21059369.
- ^ Pattanaargson, Supason; Munhapol, Thitinun; Hirunsupachot, Piyawan; Luangthongaram, Pamornwan (30 January 2004). "Photoisomerization of octyl methoxycinnamate". Journal of Photochemistry and Photobiology A: Chemistry. 161 (2): 269–274. doi:10.1016/S1010-6030(03)00282-X. ISSN 1010-6030.
- ^ Sharma, Anežka; Bányiová, Katarína; Babica, Pavel; El Yamani, Naouale; Collins, Andrew Richard; Čupr, Pavel (2017). "Different DNA damage response of cis and trans isomers of commonly used UV filter after the exposure on adult human liver stem cells and human lymphoblastoid cells". Science of the Total Environment. 593–594: 18–26. Bibcode:2017ScTEn.593...18S. doi:10.1016/j.scitotenv.2017.03.043. PMID 28340478.
- ^ Nakajima, Mariko; Kawakami, Tsuyoshi; Niino, Tatsuhiro; Takahashi, Yasuo; Onodera, Sukeo (2009). "Aquatic Fate of Sunscreen Agents Octyl-4-methoxycinnamate and Octyl-4-dimethylaminobenzoate in Model Swimming Pools and the Mutagenic Assays of Their Chlorination Byproducts". Journal of Health Science. 55 (3): 363–372. doi:10.1248/jhs.55.363. ISSN 1344-9702.
- ^ Bever, Lindsey (3 May 2018), "Hawaii might be about to ban your favorite sunscreen to protect its coral reefs", The Washington Post, retrieved 3 May 2018.
- ^ Galamgam, Jayden; Linou, Natalia; Linos, Eleni (1 November 2018). "Sunscreens, cancer, and protecting our planet". The Lancet Planetary Health. 2 (11): e465–e466. doi:10.1016/S2542-5196(18)30224-9. PMID 30396433.
- ^ McGrath, Matt (1 November 2018). "Coral: Palau to ban sunscreen products to protect reefs". BBC. Retrieved 2 November 2018.
- ^ S. Pattanaargson, T. Munhapol, P. Hirunsupachot, P. Luangthongaram (ed.): Photoisomerization of octyl methoxycinnamate. In: Journal of Photochemistry and Photobiology A: Chemistry , Elsevier Verlag, vol. 161, no. 2-3, 30 January 2004, pp. 269-274.
- ^ Process for producing 2-ethylhexanol: CL = DE 3530839A1, 29 August 1985; EP 0216151 B1, 20 August 1986.
