 | |
| Clinical data | |
|---|---|
| Routes of administration | Oral |
| ATC code |
|
| Pharmacokinetic data | |
| Elimination half-life | 1.5–1.8 hours |
| Identifiers | |
| |
| CAS Number | |
| PubChem CID | |
| IUPHAR/BPS | |
| ChemSpider | |
| UNII | |
| KEGG | |
| ChEBI | |
| ChEMBL | |
| CompTox Dashboard (EPA) | |
| ECHA InfoCard | 100.133.676 |
| Chemical and physical data | |
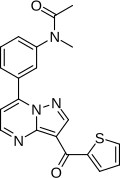
| Formula | C20H16N4O2S |
| Molar mass | 376.43 g·mol−1 |
| 3D model (JSmol) | |
| |
| |
| | |
Indiplon (INN and USAN) is a nonbenzodiazepine, hypnotic sedative that was developed in two formulations—an immediate-release formulation for sleep onset, and a modified-release (also called controlled-release or extended-release) version for sleep maintenance.
Pharmacology[edit]
Pharmacodynamics[edit]
Indiplon works by enhancing the action of the inhibitory neurotransmitter GABA, like most other nonbenzodiazepine sedatives. It primarily binds to the α1 subunits of the GABAA receptors in the brain.[1]
Pharmacokinetics[edit]
Indiplon has a short elimination half-life of 1.5 to 1.8 hours in young and elderly subjects, respectively.[2]
History[edit]
Indiplon was discovered at Lederle Laboratories (which was later acquired by Wyeth) in the 1980s and was called CL 285,489.[3]: 454 In 1998 Lederle licensed it, along with other early stage drug candidates, to DOV Pharmaceutical, a startup formed by former Lederle employees, and Dov exclusively sublicensed its rights in the drug to Neurocrine Biosciences in that same year.[3] In 2002, Neurocrine entered into an agreement with Pfizer to develop the drug.[3]
Indiplon was originally scheduled for release in 2007, when Sanofi-Aventis' popular hypnotic zolpidem lost its patent rights in the United States and thus became available as a much less expensive generic. In 2002, Neurocrine Biosciences had entered into an agreement with Pfizer to co-market indiplon in the US, in a deal worth a potential $400mn.[4] However, following the issuing of a non-approvable letter for the modified-release 15 mg formulation and an approvable letter with stipulations for the 5 mg and 10 mg immediate-release version by the FDA in May 2006,[5] Pfizer ended its relationship with Neurocrine.[6] Neurocrine's stock price dropped 60% on the news.[7]
Following a resubmission, the FDA in December 2007 deemed Neurocrine's new drug application (NDA) 'approvable' in the 5 and 10 mg formulations,[8] but requested new studies as a prerequisite to approval, including a clinical trial in the elderly, a safety study comparing adverse effects to those of similarly marketed drugs, and a preclinical study examining indiplon's safety in the third trimester of pregnancy.[9]
Following the 2007 FDA letter, Neurocrine decided to discontinue all clinical and marketing development of Indiplon in the United States.[8][9]
References[edit]
- ^ Petroski RE, Pomeroy JE, Das R, Bowman H, Yang W, Chen AP, Foster AC (April 2006). "Indiplon is a high-affinity positive allosteric modulator with selectivity for alpha1 subunit-containing GABAA receptors" (PDF). The Journal of Pharmacology and Experimental Therapeutics. 317 (1): 369–77. doi:10.1124/jpet.105.096701. PMID 16399882. S2CID 46510829.
- ^ Lemon MD, Strain JD, Hegg AM, Farver DK (September 2009). "Indiplon in the management of insomnia". Drug Design, Development and Therapy. 3: 131–142. doi:10.2147/dddt.s3207. PMC 2769245. PMID 19920929.
- ^ a b c Neubauer DN (2010). "Indiplon". In Monti JS, Pandi-Perumal SR, Möhler H (eds.). GABA and Sleep: Molecular, Functional and Clinical Aspects. Springer Science & Business Media. pp. 453–464. ISBN 9783034602266.
- ^ "San Diego's Neurocrine Biosciences Scores Second Big Deal in Two Days". The Motley Fool. 18 June 2010.
- ^ "Neurocrine's FDA Nightmare". TheStreet.com. 16 May 2006.
- ^ "Pfizer Drops Neurocrine Deal". TheStreet.com. 22 June 2006.
- ^ "Neurocrine stock price plunges 60 percent:FDA's mixed review of sleeping pill Indiplon could threaten Pfizer-Neurocrine partnership". CNN Money. 15 May 2006.
- ^ a b "Neurocrine Receives Approvable Letter for Indiplon Capsules with Additional Safety and Efficacy Data Required by FDA" (Press release). Neurocrine Biosciences, Inc. 2007-12-13. Retrieved 2007-12-13.
- ^ a b "Additional Pipeline Projects". Neurocrine. 2012-02-16. Archived from the original on 2012-03-27. Retrieved 2014-06-24.