graff= 31095783 | doi = 10.1002/adma.201901418 }}</ref> Such a model can be further extended to the cases of liquid-solid, liquid-liquid and even gas-liquid interactions.[1]
Triboelectric series
| Triboelectric series: |
| Most positively charged |
| + |
| Hair, oily skin |
| Nylon, dry skin |
| Glass |
| Acrylic, Lucite |
| Leather |
| Rabbit's fur |
| Quartz |
| Mica |
| Lead |
| Cat's fur |
| Silk |
| Aluminium |
| Paper (Small positive charge) |
| Cotton |
| Wool (No charge) |
| 0 |
| Steel (No charge) |
| Wood (Small negative charge) |
| Amber |
| Sealing wax |
| Polystyrene |
| Rubber balloon |
| Resins |
| Hard rubber |
| Nickel, copper |
| Sulfur |
| Brass, silver |
| Gold, platinum |
| Acetate, rayon |
| Synthetic rubber |
| Polyester |
| Styrene and polystyrene |
| Orlon |
| Plastic wrap |
| Polyurethane |
| Polyethylene (like Scotch tape) |
| Polypropylene |
| Vinyl (PVC) |
| Silicon |
| Teflon (PTFE) |
| Silicone rubber |
| Ebonite |
| − |
| Most negatively charged |
A triboelectric series is a list of materials, ordered by certain relevant properties, such as how quickly a material develops a charge relative to other materials on the list. Johan Carl Wilcke published the first one in a 1757 paper on static charges.[2][3] Materials are often listed in order of the polarity of charge separation when they are touched with another object. A material towards the bottom of the series, when touched to a material near the top of the series, will acquire a more negative charge. The farther away two materials are from each other on the series, the greater the charge transferred. Materials near to each other on the series may not exchange any charge, or may even exchange the opposite of what is implied by the list. This can be caused by rubbing, by contaminants or oxides, or other variables. The series was further expanded by Shaw[4] and Henniker[5] by including natural and synthetic polymers, and showed the alteration in the sequence depending on surface and environmental conditions. Lists vary somewhat as to the exact order of some materials, since the relative charge varies for nearby materials. From actual tests, there is little or no measurable difference in charge affinity between metals, probably because the rapid motion of conduction electrons cancels such differences.[6]
Another triboelectric series based on measuring the triboelectric charge density of materials was quantitatively standardized by Prof. Zhong Lin Wang's group.[7] The triboelectric charge density of the tested materials was measured with respect to a liquid metal in a glove box under well-defined conditions, with fixed temperature, pressure and humidity to achieve reliable values. The proposed method standardizes the experimental set up for uniformly quantifying the surface triboelectrification of general materials.

Cause
Although the part 'tribo-' comes from the Greek for "rubbing", τρίβω (τριβή: friction), the two materials only need to come into contact for electrons to be exchanged. After coming into contact, a chemical bond is formed between parts of the two surfaces, called adhesion, and charges move from one material to the other to equalize their electrochemical potential. This is what creates the net charge imbalance between the objects. When separated, some of the bonded atoms have a tendency to keep extra electrons, and some a tendency to give them away, though the imbalance will be partially destroyed by tunneling or electrical breakdown (usually corona discharge). In addition, some materials may exchange ions of differing mobility, or exchange charged fragments of larger molecules.
The triboelectric effect is related to friction only because they both involve adhesion. However, the effect is greatly enhanced by rubbing the materials together, as they touch and separate many times.[8]
For surfaces with differing geometry, rubbing may also lead to heating of protrusions, causing pyroelectric charge separation which may add to the existing contact electrification, or which may oppose the existing polarity. Surface nano-effects are not well understood, and the atomic force microscope has enabled rapid progress in this field of physics.
Sparks
Because the surface of the material is now electrically charged, either negatively or positively, any contact with an uncharged conductive object or with an object having substantially different charge may cause an electrical discharge of the built-up static electricity: a spark. A person simply walking across a carpet, removing a nylon[citation needed] shirt or rubbing against a car seat can also create a potential difference of many thousands of volts, which is enough to cause a spark one millimeter long or more.
Electrostatic discharge may not be evident in humid climates because surface condensation normally prevents triboelectric charging, while increased humidity increases the electrical conductivity of the air.
Electrostatic discharges (other than lightning which comes from triboelectric charging of ice and water droplets within clouds) cause minimal harm because the energy (1/2V2C) of the spark is very small, being typically several tens of micro joules in cold dry weather, and much less than that in humid conditions; however, such sparks can ignite flammable vapors (see risks and counter-measures). This is not the case when the capacitance of one of the objects is very large.
Mechanism of triboelectrification
Interatomic interaction potential can be applied to understand the interactions between atoms. When two atoms are at equilibrium positions, with an equilibrium interatomic distance, the electron clouds or wave functions are overlapped partially. On one hand, if the two atoms get close to each other as pressed by an external force, the interatomic distance becomes shorter than the equilibrium distance, the two atoms thus repel each other because of the increase in electron cloud overlap. It is in this region that electron transfer occurs. On the other hand, if the two atoms are separated from each other such that they have a larger interatomic distance than the equilibrium distance, they will attract with each other due to long-range Van der Waals interaction.
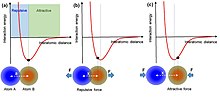
An atomic-scale charge transfer mechanism (generic electron-cloud-potential model) was proposed for the triboelectrification.[9][10] First, before the atomic-scale contact of two materials, there is no overlap between their electron clouds, and an attractive force exists. The electrons are so tightly bound in specific orbits so that they cannot escape freely. Then, when the two atoms in two materials get close to contact, an ionic or covalent bond is formed between them by the electron cloud overlap. An external force can further decrease the interatomic distance (bond length), and the strong electron cloud overlap induces the drop of the energy barrier between the two, resulting in electron transfer, which is the triboelectrification process. Once the two atoms are separated, the transferred electrons would remain because an energy is needed for the electrons to transfer back, forming the electrostatic charges on surfaces of the materials.

In aircraft and spacecraft
Aircraft flying in weather will develop a static charge from air friction on the airframe. The static can be discharged with static dischargers or static wicks.
NASA follows what they call the "triboelectrification rule" whereby they will cancel a launch if the launch vehicle is predicted to pass through certain types of clouds. Flying through high-level clouds can generate "P-static" (P for precipitation), which can create static around the launch vehicle that will interfere with radio signals sent by or to the vehicle. This may prevent transmitting of telemetry to the ground or, if the need arises, sending a signal to the vehicle, particularly critical signals for the flight termination system. When a hold is put in place due to the triboelectrification rule, it remains until Space Wing and observer personnel, such as those in reconnaissance aircraft, indicate that the skies are clear.[11]
Risks and counter-measures
Ignition
The effect is of considerable industrial importance in terms of both safety and potential damage to manufactured goods. Static discharge is a particular hazard in grain elevators owing to the danger of a dust explosion. The spark produced is fully able to ignite flammable vapours, for example, petrol, ether fumes as well as methane gas. For bulk fuel deliveries and aircraft fueling a grounding connection is made between the vehicle and the receiving tank prior to opening the tanks. When fueling vehicles at a retail station touching metal on the car before opening the gas tank or touching the nozzle may decrease one's risk of static ignition of fuel vapors.[citation needed]
In the workplace
Means have to be provided to discharge static from carts which may carry volatile liquids, flammable gasses, or oxygen in hospitals. Even where only a small charge is produced, it can result in dust particles being attracted to the rubbed surface. In the case of textile manufacture this can lead to a permanent grimy mark where the cloth comes in contact with dust accumulations held by a static charge. Dust attraction may be reduced by treating insulating surfaces with an antistatic cleaning agent.
Damage to electronics
Some electronic devices, most notably CMOS integrated circuits and MOSFET transistors, can be accidentally destroyed by high-voltage static discharge. Such components are usually stored in a conductive foam for protection. Grounding oneself by touching the workbench, or using a special bracelet or anklet is standard practice while handling unconnected integrated circuits. Another way of dissipating charge is by using conducting materials such as carbon black loaded rubber mats in operating theatres, for example.
Devices containing sensitive components must be protected during normal use, installation, and disconnection, accomplished by designed-in protection at external connections where needed. Protection may be through the use of more robust devices or protective countermeasures at the device's external interfaces. These may be opto-isolators, less sensitive types of transistors, and static bypass devices such as metal oxide varistors.
See also
References
- ^ Nie J, Wang Z, Ren Z, Li S, Chen X, Lin Wang Z (May 2019). "Power generation from the interaction of a liquid droplet and a liquid membrane". Nature Communications. 10 (1): 2264. Bibcode:2019NatCo..10.2264N. doi:10.1038/s41467-019-10232-x. PMC 6531479. PMID 31118419.
- ^ A Natural History: Devin Corbin | The Owls
- ^ Gillispie CC (1976). Dictionary of Scientific Biography. New York: Scribner. pp. 352–353.
- ^ Fowle FE (1921). Smithsonian Physical Tables. Washington: Smithsonian Institution. p. 322.
- ^ Henniker J (November 1962). "Triboelectricity in Polymers". Nature. 196 (4853): 474. Bibcode:1962Natur.196..474H. doi:10.1038/196474a0. S2CID 4211729.
- ^ The TriboElectric Series
- ^ a b Zou H, Zhang Y, Guo L, Wang P, He X, Dai G, et al. (March 2019). "Quantifying the triboelectric series". Nature Communications. 10 (1): 1427. Bibcode:2019NatCo..10.1427Z. doi:10.1038/s41467-019-09461-x. PMC 6441076. PMID 30926850.
- ^ Cite error: The named reference
:2was invoked but never defined (see the help page). - ^ Lowell J (1 December 1977). "The role of material transfer in contact electrification". Journal of Physics D: Applied Physics. 10 (17): L233–L235. Bibcode:1977JPhD...10L.233L. doi:10.1088/0022-3727/10/17/001. ISSN 0022-3727.
- ^ Kanigan, Dan (27 October 2009). "Flight Rules and Triboelectrification (What the Heck is That?) | Ares I-X Test Flight". NASA. Retrieved 31 January 2017.
Further reading
- Besançon RM (1985). The Encyclopedia of Physics, Third Edition. Van Nostrand Reinhold Company. ISBN 978-0-442-25778-1.
- Allen RC (November 2000). "Triboelectric generation: getting charged". EE-Evaluation Engineering. 39 (11): S4–+.
External links
- The TriboElectric Series (great detail)
- Video: Detailed explanation by professional physicists
- Charged Rod Demonstration, University of Minnesota
- NASA, Science Crackling Planets
- A plastic comb rubbed with a cotton cloth attracts small pieces of paper (video)
- BBC News Article, 2005 - Man's static jacket sparks alert
- Triboelectric Generation: Getting Charged