| Huntington's disease | |
|---|---|
| Specialty | Neurology |
| Frequency | 0.0123% (United Kingdom) |
| Huntington's disease | |
|---|---|
| Specialty | Neurology |
| Frequency | 0.0123% (United Kingdom) |
Huntington's disease (HD, also Huntington disease), formerly known as Huntington's chorea, is a rare inherited genetic disorder characterized by abnormal body movements called chorea, and a reduction of various mental abilities. It takes its name from the Ohio physician George Huntington who described it precisely in 1872.
Huntington's disease is caused by a rare trinucleotide repeat expansion in the Huntingtin gene. Huntington's disease shows a variable age of onset of physical symptoms, generally between 40 and 50 years old.
Symptoms and signs
The symptoms of Huntington’s disease occur gradually over time - there is no sudden loss of abilities and it is hard to determine when symptoms initially occur. Exact symptoms can vary greatly from person to person, some having an acute version of a symptom and another only mild, if at all, but most are generally somewhere in between.
Symptoms of the disorder are of three types: physical, cognitive and psychiatric.
Physical
- General lack of coordination and involuntary movements causing an unsteady gait.
- Most people with HD eventually exhibit chorea, which is jerky, random, uncontrollable, rapid movements, although some exhibit very slow movement and stiffness (bradykinesia, dystonia). Typically, the abnormal movements begin at the extremities and then later progress.
- Loss of facial expression, called "masks in movement".
- The ability to synchronise muscles to speak and swallow is also affected.
- Weight Loss.
Cognitive
Some selective areas of cognitive ability are impaired, whereas others remain intact. Areas affected are:
- Executive function - planning, cognitive flexibility, abstract thinking, rule acquisition, initiating appropriate actions and inhibiting inappropriate actions.
- Psychomotor function - slowing of thought processes to control muscles
- Language abilities are not impaired but actual speech is.
- Perceptual and spatial skills of self and surrounding environment.
- Memory - ability to select correct method of remembering new information, but not actual memory itself.
- Learning new skills is affected - depending on which parts of the brain the skill requires.
Psychiatric
These vary more than cognitive and motor symptoms and may be any of the following:
- Blunting - reduced display of emotions
- Egocentrism
- Anxiety
- Depression
- Aggression
- Compulsions, which can often lead to addictions such as alcoholism and gambling
- Hypersexuality
Diagnosis
To determine if someone has the initial symptoms, a physical and/or psychological examination is required. The uncontrollable movements are often the symptoms which cause initial alarm and lead to diagnosis; however, the disease may begin with cognitive or emotional symptoms, which are not always recognized.
Every child of a person with Huntington's Disease is "at-risk", which means they have a fifty percent chance of inheriting the gene and the disease. Pre-symptomatic testing is possible by means of a blood test which counts the number of repetitions in the gene. A negative blood test means that the individual does not carry the gene, will never develop symptoms, and cannot pass it on to children. If the test is positive, then the individual will develop the disease at some point in the future, assuming he or she lives long enough to do so. A pre-symptomatic positive blood test is not considered a diagnosis, since it may be decades before onset.
Because of the ramifications on the life of an at-risk individual, with no cure for the disease and no proven way of slowing it, several counseling sessions are usually required before the blood test. Unless a child shows significant symptoms of the juvenile form, children under eighteen cannot be tested. The Huntington's Disease Society of America strongly encourages these restrictions in their testing protocol. A pre-symptomatic test is a life-changing event and a very personal decision. For those living in America, there is a list of testing centers available on the HDSA homepage. [1]
In vitro fertilisation and embryonic genetic screening are also possible, enabling gene-positive or at-risk individuals the option of making sure their children will be clear of the disease. Expense and the ethical considerations of abortion are potential drawbacks to these procedures.
The full pathological diagnosis is established by neurological examination findings and/or demonstration of cell loss, especially in the caudate nucleus, supported by a cranial CT or MRI scan findings.
About 10 percent of Huntington's disease cases occur in people under the age of 20 years. This is referred to as Juvenile Huntington’s disease, or sometimes as "akinetic-rigid" or "Westphal variant" HD.
Pathophysiology
The normal function of Huntingtin is unclear. It has however been experimentally demonstrated that Huntingtin acts as a transcription factor in upregulating the expression of Brain-derived neurotrophic factor (BDNF). In the deficient protein, there is supression of this transcription regulatory function of Huntingtin and hence underexpression of BDNF. Various studies have shown possible links between low levels of BDNF and conditions such as depression, Obsessive-compulsive disorder and dementia, allthough it is still not known whether these levels represent a cause or a symptom.
Degeneration of the striatum (a part of the brain consisting of the caudate nucleus and the putamen) can be found. There is also neuronal loss and astrogliosis, as well as loss of medium spiny neurons, a GABAergic result. Intranuclear inclusions that stain for ubiquitin and huntingtin can be seen, as well as huntingtin in cortical neurites. Genetically, huntingtin is found on chromosome 4, as are CAG repeats. It is suspected that the cross-linking of huntingtin results in aggregates which are toxic, and can lead to dysfunction of the proteosome system. This mitochondrial dysfunction can lead to excitotoxicity and oxidative stress.
Linkage between CAG repeats (huntingtin) and mitochondrial failure, however, is far from clear. There is some evidence that aggregates may trap critical enzymes that are involved in energy metabolism.
While theories as to how the mutation brings about disease remain diverse and speculative, researchers have identified many specific subcellular abnormalities associated with the mutant protein, as well as unusual properties of the protein in vitro. Just as one example, in 2002, Max Perutz, et al discovered that the glutamine residues form a nanotube[2] in vitro, and the mutated forms are long enough in principle to pierce cell membranes.
Genetics
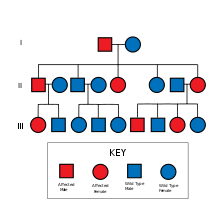
The causative gene for Huntington's disease, HD, is located on chromosome 4. Huntington's disease is inherited in an autosomal dominant fashion. That is, a recipient of the gene only needs one allele from one parent, to inherit the disease. More often, genetic diseases are autosomal recessive, meaning that they need two alleles, one from each parent, to inherit the disease. The dominant nature of Huntington's disease increases the chance of the disease occurring in offspring. A parent who has the disorder has a 50% chance of passing on the gene to each child.
When the gene has more than 35 repeats, the replication process becomes unstable and the number of repeats can change in successive generations. If the gene is inherited from the mother the count is usually similar, but tends to increase if inherited from the father.[3] Because of the progressive increase in length of the repeats, the disease tends to increase in severity and present at an earlier age in successive generations. This is known as anticipation.
The product of this gene is a 348 kDa cytoplasmic protein called huntingtin. The continuous aggregation of huntingtin molecules in neuronal cells gives rise to cell death, especially in the frontal lobes and the basal ganglia (mainly in the caudate nucleus) by some unknown mechanism. Some think that the form of apoptosis is the splitting of the lysosome so that the hydrolytic enzymes within are released. This will cause the cell membrane to be split and the cell to die. Huntingtin has a characteristic sequence of fewer than 40 glutamine amino acid residues (encoded by CAG trinucleotide repeats) in the normal form; the mutated huntingtin causing the disease has more than 40 residues. The severity of the disease is generally proportional to the number of extra residues.
Management
Although there is no treatment to fully stop the progression of the disease, there are treatments available to help control the chorea, although these may have the side effect of aggravating bradykinesia or dystonia.
Other standard treatments to alleviate emotional symptoms include the use of antidepressants and sedatives, with antipsychotics (in low doses) for psychotic symptoms. Care needs to be taken with antipsychotic usage as people suffering psychotic symptoms of organic origin are often more sensitive to the side effects of these drugs.
Nutrition is an important part of treatment, most HD sufferers need two to three times the calories than the average person to maintain body weight, so a nutritionist's advice is needed. Note: an average calorie intake is between 2000 (women) to 2500 (children and men).
Speech therapy can help by improving speech and swallowing methods. This advice should be sought early on, as the ability to learn new things is reduced as the disease progresses.
To aid swallowing thickener can be added to drinks. When swallowing becomes hazardous the option of using a stomach PEG for intake of nutrients is often chosen, this reduces the chances of pnuemonia due to aspiration of food.
In the June 16, 2006 issue of Cell, scientists at the University of British Columbia (UBC) and Merck Labs presented findings that the neurodegeneration caused by mutant huntingtin (htt) is related to caspase-6 cleaving the enzyme. Transgenic mice that have caspase-6 resistant huntingtin did not show effects of the huntingtin enzyme.[4] The researchers found "substantial support for the hypothesis that cleavage at the caspase-6 site in mhtt [mutant huntingtin] represents a crucial rate-limiting event in the pathogenesis of HD.... Our study highlights the importance of preventing cleavage of htt at this site and also reinforces the importance of modulating excitotoxicity as a potential therapeutic approach for HD."
Prospective
Gene silencing
The most hopeful prospective treatment currently studied is based on gene silencing. Since HD is caused by expression of a single gene, silencing of the gene could theoretically halt the progression of the disease. One study with a mouse model of HD treated with siRNA therapy achieved 60% knockdown in expression of the defective gene. Progression of the disease halted.[5] Additional research shows full recovery of motor function in late stage Tet/HD94 mice after addition of doxycycline.[6]
Calorie restriction
It has been shown that a calorie restrictive diet delays the onset of symptoms in HD mice.[7]
Omega-III EPA
EPA, an Omega-III fatty acid, has been shown to slow and possibly reverse the progression of the disease. It is currently in FDA clinical trial, as Miraxion© (LAX-101), for prescription use. Clinical trials utilize 2 grams per day of EPA. In the United States, it is available over the counter in lower concentrations in Omega-III and fish oil supplements.
Others
Trials and research are conducted on Drosophila fruit flies and mice that have been gentically modified to exhibit HD, before moving on to human trials.
Other agents and measures that have shown promise in initial experiments include dopamine receptor blockers, creatine, CoQ10, the antibiotic Minocycline, Trehalose, exercise, antioxidant-containing foods and nutrients, antidepressants (notably, but not exclusively, selective serotonin reuptake inhibitors SSRIs, such as sertraline, fluoxetine, and paroxetine) and select Dopamine antagonists, such as Tetrabenazine.
In 2004 it was found that a simple sugar called trehalose can alleviate symptoms in genetically modified mice.
Pig cell implants in Huntington's Disease trial: Living Cell Technologies in New Zealand has attempted trials with positive results in primates [8], but is yet to conduct a human trial.
This research is reviewed on various websites for Huntington's sufferers and their families, including the Huntington's Disease Lighthouse, Hereditary Disease Foundation, and Stanford HOPES websites. Primary research can be found by searching the National Library of Medicine's PubMed.
There are clinical trials of various treatments still to be completed, or started. For example the US registar of trials has 9 that are recruiting volunteers [9].
The Folding@home project is the second largest distributed processing project on the internet It models protein folding and HD is listed amongst the potential benefactors of its results.
Prognosis
Onset of Huntington's disease seems to be correlated to the number of CAG repeats a person has in their HD gene. Generally, the higher the number of repeats the sooner onset is. [10] The number of repeats may change slightly with each successive generation, so that the age of onset may vary as well. Symptoms of Huntington’s disease usually become noticeable in the mid 30s to mid 40s.
Juvenile HD has an age of onset anywhere between infancy and 20 years of age. The symptoms of juvenile HD are different from those of adult-onset HD in that they generally progress faster and are more likely to exhibit rigidity and bradykinesia (very slow movement) instead of chorea.
Mortality is due to infection (mostly pneumonia), injuries related to a fall, or other complications resulting from Huntington's Disease rather than the disease itself, and is usually 10 to 25 years after the onset of obvious symptoms. The suicide rate amongst HD sufferers is much greater than the national average[11]
Epidemiology
Incidence
The incidence is 5 to 8 per 100,000, varying geographically.
Ethical aspects
Huntington's disease presents individuals and families with several problems:
- Testing for the presence of the disease
- Whether to have children
- Informing children with an HD positive parent that they are at risk
- Coping with the discovery of the disease in a family member.
- Testing of grandchildren of a sufferer has serious ethical implications if their parent declines testing, as a positive result in a grandchild's test automatically diagnoses the parent.
- Coping with the social and personal impacts of impairment of learning new skills.
Genetic counseling may provide perspective for those at risk of the disease. Some choose not to undergo HD testing due to numerous concerns (for example, insurability).
For those at risk, or known to have the disease, consideration is necessary prior to having children due to the genetically dominant nature of the disease. In vitro and embryonic genetic screening now make it possible (with 99% certainty) to have an HD-free child; however, the cost of this process can easily reach tens of thousands of dollars.
Parents and grandparents recently discovered to possess the disease are left with the question of when and how to tell their children and grandchildren. The issue of disclosure also comes up when siblings are diagnosed with the disease, and especially in the case of identical twins. It is not unusual for entire segments of a family to become alienated as a result of such information or the withholding of it.
Financial institutions are also faced with the question of whether to use genetic testing results when assessing an individual, e.g. for life insurance. Some countries organisations have already agreed not to use this information.
Cultural references
Huntington's disease features prominently in Ian McEwan's 2005 novel Saturday, where the protagonist Dr Henry Perowne diagnoses his antagonist Baxter's condition. The disease is also featured in Pål Johan Karlsen's 2002 Norwegian novel Daimler, where the main character Daniel Grimsgaard is afflicted.
As well, Huntington's disease figures in an episode of the BBC drama Waterloo Road.
History
- c300 There is evidence that doctors as far back as the Middle Ages knew of this disease. It was known, amongst other conditions with abnormal movements, as St Vitus dance. St Vitus is the Christian patron saint of epileptics who was martyred in 303.
- Middle ages. People with the condition were often persecuted as being witches or as being possessed by spirits, and were shunned, exiled or worse. Some speculate that at least some of the "witches" in the Salem Witch Trials in 1692 had HD.[12]
- 1860 One of the early medical descriptions of Huntington's disease was made in 1860 by a Norwegian district physician, Johan Christian Lund. He noted that in Setesdalen, a remote and rather secluded area, there was a high prevalence of dementia associated with a pattern of jerking movement disorders that tended to run in families. This is the reason for the disease being commonly referred to as Setesdalsrykkja (Setesdalen=the location, rykkja=jerking movements) in Norwegian.
- 1872 George Huntington was one of three generations of medical practitioners in Long Island. With their combined experience of several generations of a family with the same symptoms, he realised their conditions were linked and set about describing it. A year after leaving medical school , in 1872, he presented his accurate definition of the disease to a medical society in Middleport, Ohio.
- c1923 Smith Ely Jelliffe (1866-1945) and Frederick Tilney (1875-1938) began analyzing the history of Huntington's sufferers in New England.
- 1932 P. R. Vessie expanded Jelliffe and Tilney's work, tracing about a thousand people with Huntington's back to two brothers and their families who left Bures in Essex for Suffolk bound for Boston in 1630.
- 1979 The U.S-Venezuela Huntington's Disease Collaborative Research Project began an extensive study which gave the basis for the gene to be discovered. This was conducted in the small and isolated Venezuelan fishing village of Barranquitas. Families there have a high presence of the disease, which has proved invaluable in the research of the disease.
- 1983 Professor Wexler, James Gusella, David Housman, P. Michael Conneally and their colleagues find the general location of the gene, using DNA marking methods for the first time - an important first step toward the Human Genome Project.
- 1993 The Huntington's Disease Collaborative Research Group isolates the precise gene at 4p16.3.
- 1996 A transgenic mouse was created that could be made to exhibit HD greatly advancing how much experimentation can be achieved.
- 1997 Researchers discovered that the mutant huntingtin protein bunches up (mis folds) to form nuclear inclusions.
- 2001 Christopher Ross and his team at Johns Hopkins University described how the HD gene causes the death of cells through a flawed form of the huntingtin protein.[13]
The full record of research is extensive.[14][15]
Relevant organisations
- 1967 Woody Guthrie's wife, Marjorie Guthrie, helped found the Committee to Combat Huntington's Disease, after his death whilst suffering from HD. This eventually became the Huntington's Disease Society of America[16]. Since then, lay organisations have been formed in many countries around the world.
- 1968 After experiencing Huntingtons in his wife's family, Dr. Milton Wexler was inspired to start the Hereditary Disease Foundation (HDF). Professor Nancy Wexler, Dr. Wexler's daughter, was in the research team in Venezeula and is now president of the HDF.
- 1974 the first international meeting took place when the founders of the Canadian HD Society (Ralph Walker) and of the British HD Society (Mauveen Jones) attended the annual meeting of the American HD Society
- 1977 second meeting organised by the Dutch Huntington Society the "Vereniging van Huntington", representatives of six countries were present.
- 1979 International Huntington Association (IHA) formed during international meeting in Oxford (England) organised by HDA of England.
- 1981-2001 Biennial meetings held by IHA which became the World Congress on HD.
- 2003 the first World Congress on Huntington's Disease was held in Toronto. This is a biennial meeting for associations and researchers to share ideas and research, which is held on odd-number years. The Euro-HD Network[17] was started as part of the Huntington Project[18], funded by the High-Q Foundation[19].
References
- ^ www.hdsa.org
- ^ Perutz, M.F., J.T. Finch, J. Berriman, and A. Lesk (2002). "Amyloid fibers are water-filled nanotubes". Proceedings of the National Academy of Sciences. 99: 5591–5595.
{{cite journal}}: CS1 maint: multiple names: authors list (link) - ^ RM Ridley, CD Frith, TJ Crow and PM Conneally (1988). "Anticipation in Huntington's disease is inherited through the male line but may originate in the female". Journal of Medical Genetics. 25: 589–595.
{{cite journal}}: CS1 maint: multiple names: authors list (link) - ^ Graham, RK, Y Deng, EJ Slow, B Haigh, N Bissada, G Lu, J Pearson, J Shehadeh, L Bertram, Z Murphy, SC Warby, CN Doty, S Roy, CL Wellington, BR Leavitt, LA Raymond, DW Nicholson, MR Hayden (2006-06-16). "Cleavage at the Caspase-6 site is required for neuronal dysfunction and degeneration due to mutant Huntingtin". Cell. 125: 1179–1191.
{{cite journal}}: CS1 maint: multiple names: authors list (link) - ^ Sirna Therapeutics. "Huntington's Disease Overview". Retrieved 2006-07-16.
- ^ Miguel Díaz-Hernández, Jesús Torres-Peraza, Alejandro Salvatori-Abarca, María A. Morán, Pilar Gómez-Ramos, Jordi Alberch, and José J. Lucas (October 19, 2005). "Full Motor Recovery Despite Striatal Neuron Loss and Formation of Irreversible Amyloid-Like Inclusions in a Conditional Mouse Model of Huntington's Disease". The Journal of Neuroscience. 25 (42): 9773–9781. Retrieved 2006-07-16.
{{cite journal}}: CS1 maint: multiple names: authors list (link) - ^ Fasting Forestalls HUNTINGTON'S DISEASE in Mice on friendsoffreedom.org
- ^ World health Article
- ^ clinicaltrials.gov
- ^ The Huntington Disease lighthouse.org
- ^ http://www.huntington-assoc.com/Critical%20ab05.pdf]
- ^ The brief history of HD on stanford.edu
- ^ Huntington’s disease on pubs.acs.org
- ^ Achievements of Hereditary Disease Foundation
- ^ HDA research news - medical research into treatment & prevention on hda.org.uk
- ^ Huntington's Disease Society of America
- ^ Euro-HD Network
- ^ Huntington Project
- ^ High-Q Foundation
Bibliography
- John P. Conomy, M.D., J.D. "Dr. George Sumner Huntington and the Disease Bearing His Name".
{{cite web}}: CS1 maint: multiple names: authors list (link)
- P. R. Vessie (1932). "On the transmission of Huntington's chorea for 300 years – the Bures family group". Journal of Nervous and Mental Disease, Baltimore. 76: 553–573.
- G. Huntington (1872-04-13). "On Chorea". Medical and Surgical Reporter of Philadelphia. 26 (15): 317–321.
{{cite journal}}: Check date values in:|date=(help)
- Gillian Bates, Peter Harper, Lesley Jones (2002). Huntington's Disease - Third Edition. Oxford: Oxford University Press. ISBN 0-19-851060-8.
{{cite book}}: CS1 maint: multiple names: authors list (link)
External links
- Huntington Project - worldwide umbrella organisation for the clinical research efforts for HD.
- Huntington Study Group
- Huntington's Disease Research - Recent research abstracts on Huntington's Disease.
- Article 143100 - Huntington Disease , Online Mendelian Inheritance in Man, Johns Hopkins University
- Article 606438 - Huntingtons Disease-Like 2 Online Mendelian Inheritance in Man, Johns Hopkins University
- Huntington's Outreach Project for Education, at Stanford (HOPES) - A layperson's guide to Huntington's disease
- History of Medicine Bulletin,The Johns Hopkins University, a biography of George Huntington and other relevant information.
- Worldwide Education and Awareness for Movement Disorders - HD section
Lay organisations
- The International Huntington Association - coordinates HD organisations in 39 countries with contacts in others across the world.
- Huntington's Disease Society of America
- Hereditary Disease Foundation - spearheaded Venezuela Collaborative Huntington's Disease Project.
- European HD Network
- Huntington's Disease Support Club
- Australian Huntington's Disease Association (NSW) Inc.
- Huntington's Disease Lighthouse
- Huntington's Disease Association UK
- Huntington Liga BE