
| |
| Names | |
|---|---|
| Preferred IUPAC name
S-[(tert-Butylsulfanyl)methyl] O,O-diethyl phosphorodithioate | |
Other names
| |
| Identifiers | |
3D model (JSmol)
|
|
| ChemSpider | |
| ECHA InfoCard | 100.032.679 |
PubChem CID
|
|
| UNII | |
CompTox Dashboard (EPA)
|
|
| |
| |
| Properties[1] | |
| C9H21O2PS3 | |
| Molar mass | 288.42 g·mol−1 |
| Appearance | clear, colorless to pale yellow or reddish-brown liquid |
| Density | 1.105 g/mL (at 24 °C) |
| Melting point | −29.2 °C (−20.6 °F; 244.0 K) |
| Boiling point | 69 °C (156 °F; 342 K) |
| < 1 mg/mL | |
| Solubility | Freely soluble in organic compounds |
| Vapor pressure | 0.0003 mmHg |
| Hazards[3] | |
| GHS labelling: | |
  
| |
| Danger | |
| H227, H300, H310, H330, H361, H370, H372, H410 | |
| P201, P202, P210, P233, P235, P260, P262, P264, P270, P271, P273, P280, P281, P284, P301, P302, P304, P307, P308, P310, P311, P313, P314, P320, P321, P322, P330, P340, P350, P361, P363, P370, P378, P391, P403, P405, P501 | |
| NFPA 704 (fire diamond) | |
| Flash point | 88 °C (190 °F; 361 K) |
| Lethal dose or concentration (LD, LC): | |
LD50 (median dose)
|
Rat (male), Oral LD50 = 13 mg/kg
Rat (female), Oral LD50 = 8 mg/kg Rat (male), Dermal LD50 = 123 mg/kg Rat (female), Dermal LD50 = 71 mg/kg |
LC50 (median concentration)
|
Rat (male), Inhalation (4 hr): 0.017 mg/L
Rat (female), Inhalation(4 hr): 0.015 mg/L Rat (male), Inhalation (1 hr calculated): 0.068 mg/L Rat (female), Inhalation (1 hr): 0.06 mg/L |
| NIOSH (US health exposure limits): | |
PEL (Permissible)
|
0 ppm [2] |
REL (Recommended)
|
0 ppm [2] |
| Safety data sheet (SDS) | [1] |
| Pharmacology[4] | |
| |
| Pharmacokinetics: | |
| Urine (86.9 - 96 %) | |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa).
| |
Terbufos is a chemical compound used in insecticides and nematicides. It is part of the chemical family of organophosphates. It is a clear, colourless to pale yellow or reddish-brown liquid and sold commercially as granulate.[5]
History[edit]
Terbufos is used on various crops including bananas, beans, citrus, coffee, groundnuts, sorghum, potatoes, sunflowers and maize as soil cover to combat wireworms, mossy beetles, beet flies and the black bean louse.[6][7] It is not approved for use in the European Union.[8] Also the World Health Organization classifies terbufos as a class Ia compound, meaning that terbufos is extremely hazardous.[9] The maximum residue limit in the European Union is 0.01 mg/kg terbufos for most crops and animal products.[10] The compound was first registered in 1974 in the United States, together with a United States patent of organophosphates for use in corn fields to deter corn rootworms.[11][12] Between 1987 and 1996, an average of about 7.5 million pounds (about 3,400 tons) of the compound was used each year.[13] In November 2006, BASF sold its global Terbufos insecticide business to American AMVAC (American Vanguard Corporation).[14]
Organophosphate poisoning is not common in the developed world. Mst cases of terbufos poisoning occur in the developing world, where protection against pesticides is scarce, but compounds such as terbufos are widespread, uncontrolled by a government and readily available for farmers.[15]
Available forms[edit]
Terbufos is available in granules for application in the agricultural sector. The compound is applied at planting in a band or on the seed furrow directly.[16]
Structure and reactivity[edit]
Structure[edit]
Terbufos, also known as S-((tert-butylthio)methyl) O,O-diethyl phosphorodithioate,[17] is a compound classified as an organophosphate. Terbufos consists of a central phosphorus atom, surrounded by four different groups. This central atom is surrounded by two ethoxy groups, one double-bonded sulfur atom and a (tert-butylthio)methanethiol group.
Reactivity[edit]
Terbufos is practically insoluble in water, but can be dissolved freely in organic compounds. It decomposes after prolonged heating at 120 °C[18][19] and can be hydrolysed when exposed to strong bases (pH>9) and acids (pH<2).[19]
Synthesis[edit]
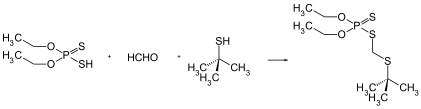
The three main compounds that are used for producing terbufos are diethyl-phosphorodithioic acid, formaldehyde and tert-butylthiol. Formaldehyde acts as a carbon donor between the diethyl-phosphorodithioic acid and tert-butylthiol groups in order to link them.[5] A secondary product of this reaction is water.
Mechanism of action[edit]
Terbufos, like other organophosphates, inactivates the acetylcholinesterase in humans by phosphorylation of the hydroxyl group of serine present at the active site of the enzyme.[20]
Metabolism[edit]
Metabolism in animals[edit]
The major excretion route of terbufos in lactating goats is via the urine. 96.0% and 86.9% of the administered compound was excreted through this route. Neither terbufos, nor a phosphorylated metabolite of the compound was found in milk, and no phosphorylated metabolite was detected in the tissues.[4] A low concentration of terbufos was detected in the liver and the kidneys. Terbufos is extensively metabolised, judging from the low levels of terbufos and its metabolites detected in goat tissues. A proposed metabolism pathway of terbufos suggests a hydrolysis of the thiolophosphorus bond, an enzymatic S-methylation, desulfuration and sulfoxidation occurred in succession.[4]
In rats, the proposed metabolism pathway includes more steps, while the metabolic product is the same as proposed in goats. The mechanism in rats includes extra steps in the metabolism of terbufos, and includes more metabolites.[4]
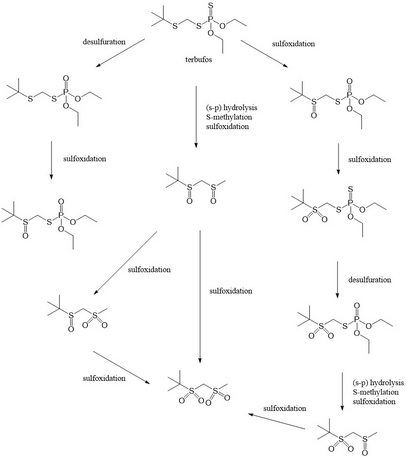
Biotransformation[edit]
Terbufos is activated by a biotransformation to a sulfone compound. This conversion can take place in the (cellular) environment but also in exposed organisms using the cytochrome P450 action. This conversion process makes the molecule much more efficient in binding with AChE. The converted form may be significantly more toxic to amphibians than the parent compound.

Toxicity[edit]
General toxicity[edit]
Terbufos can enter the body through dermal absorption (skin contact), inhalation or ingestion of the compound.[21] Studies have suggested that the human NOEL ≥0.009 mg/m^3.[22]
Metabolite toxicity[edit]
Two metabolites of terbufos have been tested for toxicity. Terbufos sulfoxide and terbufos sulfone both inhibited cholinesterase activity, but did not cause any mortalities in beagle dogs.[4]
Health effects[edit]
Acute effects[edit]
Terbufos can induce death by causing an acute cholinergic crisis (ACC). Due to the irreversible inhibition of the AChE enzyme by the compound, acetylcholine (ACh) can no longer be sufficiently broken down by the AChE. This results in excess ACh, which causes overstimulation of the neuromuscular junction. The inhibiting effect of terbufos on AChE works on the peripheral muscarinic, nicotinic synapses and central nervous system. The onset of an ACC can vary from minutes to multiple hours post-exposure.[23][20]
Symptoms of a terbufos induced ACC result in muscarinic (diaphoresis, vomiting, miosis, salivation), nicotinic (pallor and muscle weakness with respiratory failure) and CNS poisoning (headache, dizziness, altered level of consciousness) symptoms.[24] The toxic effects can be managed by early recognition of terbufos poisoning, rapid decontamination and treatment with atropine or oxime compounds.[20][25]
Long-term effects[edit]
Long term exposure effects which are specific for terbufos (effects generally associated with organophosphates (OPs) not included) are the development of lung cancer, leukemia and non-Hodgkin lymphoma (NHL) overall, as well as specific NHL subtypes. In males an increase in aggressive prostate cancer has also been observed, while in females a non-significant increase in breast cancer can be seen.[26]
No genotoxic effects were detected in vitro and in vivo. No developmental abnormalities were noted in research, but a reduced fetal body weight was observed in mammals.[4]
Detection[edit]
The amount of metabolites, caused by hydrolysis in vivo of an organophosphate compound such as terbufos, can be detected in the urine using gas chromatography and combined gas chromatography/mass spectrometry (GC-MC). Usually it is necessary to preserve the sample of the urine by addition of chloroform, to concentrate or extract the metabolites and to convert them to suitably-volatile derivatives. The detection can be useful to determine patterns of exposure. However, the levels of metabolite alone cannot be considered a guide to hazard.[27]
References[edit]
- ^ "Terbufos".
- ^ a b "OSHA Occupational Chemical Database | Occupational Safety and Health Administration".
- ^ http://www.kellysolutions.com/erenewals/documentsubmit/KellyData/VA/pesticide/MSDS/7969/241-238/241-238_COUNTER_R__15G_SYSTEMIC_INSECTICIDE_NEMATICIDE_LOCKN_LOAD_R__CLOSED_LOADING_SYSTEM_11_28_2006_4_50_14_PM.pdf[bare URL PDF]
- ^ a b c d e f g h apps.who.int/pesticide-residues-jmpr-database/Document/181
- ^ a b Unger, T.A. Pesticide Synthesis Handbook (1st ed.). p. 368.
- ^ "TERBUFOS 15 GR" (PDF). Retrieved March 22, 2018.
- ^ Heitefuss, Rudolf (2000). Pflanzenschutz : Grundlagen der praktischen Phytomedizin ; 22 Tabellen (3rd ed.). Stuttgart: Thieme. p. 397. ISBN 978-3-13-513303-4.
- ^ "EU Pesticides database - European Commission". ec.europa.eu. Retrieved 2018-03-15.
- ^ "Acutely Toxic Pesticides" (PDF).
- ^ "EU Pesticides database - European Commission". ec.europa.eu. Retrieved 2018-03-22.
- ^ "terbufos (Counter) EPA Pesticide Fact Sheet 9/88". pmep.cce.cornell.edu. Retrieved 2018-03-16.
- ^ "Methods of combatting insects and acarina using oxygenated derivatives of S-(tert-bulythio)methyl O,O-diethyl phosphorodithioate and phosphorothioate". Frank Albert Wagner, Jr., Wyeth Holdings Corp. 1974-12-19.
{{cite journal}}: Cite journal requires|journal=(help)CS1 maint: others (link) - ^ EPA: Reregistration Eligibility Decision for Terbufos (PDF; 1,3 MB), July 2006.
- ^ Pressemeldung BASF: BASF AG | BASF verkauft das globale Geschäft mit dem Insektizid Terbufos an AmVac
- ^ Liang, Y; Tong, F; Zhang, L; Li, W; Huang, W; Zhou, Y (February 2018). "Fatal poisoning by terbufos following occupational exposure". Clinical Toxicology. 56 (2): 140–142. doi:10.1080/15563650.2017.1340647. PMID 28681657. S2CID 8889797.
- ^ "EXTOXNET PIP - TERBUFOS". extoxnet.orst.edu. Retrieved 2018-03-15.
- ^ Hertfordshire, University of. "terbufos". sitem.herts.ac.uk. Retrieved 2018-03-15.
- ^ Eintrag zu Terbufos in der GESTIS-Stoffdatenbank des IFA, retrieved 1 February 2016.
- ^ a b Eintrag zu Terbufos in der Hazardous Substances Data Bank, retrieved 19 August 2012.
- ^ a b c Chowdhary, Sheemona; Bhattacharyya, Rajasri; Banerjee, Dibyajyoti (April 2014). "Acute organophosphorus poisoning". Clinica Chimica Acta. 431: 66–76. doi:10.1016/j.cca.2014.01.024. PMID 24508992.
- ^ Schulze, Larry D.; Ogg, Clyde L.; Vitzthum, Edward F. (1997). "EC97-2505 Signs and Symptoms of Pesticide Poisoning". Historical Materials from University of Nebraska-Lincoln Extension.
- ^ Bingham, E.; Cohrssen, B.; Powell, C.H.; Patty's Toxicology Volumes 1-9 5th ed. John Wiley & Sons. New York, N.Y. (2001)., p. V7 924
- ^ Lorke, Dietrich E.; Nurulain, Syed M.; Hasan, Mohamed Y.; Kuča, Kamil; Petroianu, Georg A. (October 2014). "Prophylactic administration of non-organophosphate cholinesterase inhibitors before acute exposure to organophosphates: assessment using terbufos sulfone". Journal of Applied Toxicology. 34 (10): 1096–1103. doi:10.1002/jat.2939. PMID 24136594. S2CID 41811085.
- ^ Paudyal, BP (2008). "Organophosphorus poisoning". Journal of Nepal Medical Association. 47 (172): 251–8. doi:10.31729/jnma.170. PMID 19079407.
- ^ Kassa, J. (26 November 2002). "Review of Oximes in the Antidotal Treatment of Poisoning by Organophosphorus Nerve Agents". Journal of Toxicology: Clinical Toxicology. 40 (6): 803–816. doi:10.1081/CLT-120015840. PMID 12475193. S2CID 20536869.
- ^ Lerro, Catherine C; Koutros, Stella; Andreotti, Gabriella; Friesen, Melissa C; Alavanja, Michael C; Blair, Aaron; Hoppin, Jane A; Sandler, Dale P; Lubin, Jay H; Ma, Xiaomei; Zhang, Yawei; Beane Freeman, Laura E (October 2015). "Organophosphate insecticide use and cancer incidence among spouses of pesticide applicators in the Agricultural Health Study". Occupational and Environmental Medicine. 72 (10): 736–744. doi:10.1136/oemed-2014-102798. PMC 4909328. PMID 26150671.
- ^ WHO; Environ Health Criteria 63: Organophosphorus pesticides (1986). Available from, as of July 22, 2003: http://www.inchem.org/pages/ehc.html
External links[edit]
- Terbufos in the Pesticide Properties DataBase (PPDB)
