 | |
| Clinical data | |
|---|---|
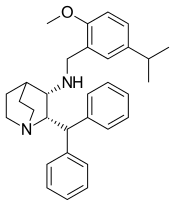
| Other names | CJ-11,974; (2S,3S)-2-Diphenylmethyl-3-[(5-isopropyl-2-methoxybenzyl)amino]quinuclidine[1] |
| Routes of administration | By mouth |
| ATC code |
|
| Legal status | |
| Legal status |
|
| Pharmacokinetic data | |
| Excretion | Urine (32%), Feces (51%) |
| Identifiers | |
| |
| CAS Number | |
| PubChem CID | |
| IUPHAR/BPS | |
| ChemSpider | |
| UNII | |
| ChEMBL | |
| Chemical and physical data | |
| Formula | C31H38N2O |
| Molar mass | 454.658 g·mol−1 |
| 3D model (JSmol) | |
| |
| |
Ezlopitant (INN,[1] code name CJ-11,974) is an NK1 receptor antagonist.[2][3][4] It has antiemetic and antinociceptive effects.[5][6] Pfizer was developing ezlopitant for the treatment of irritable bowel syndrome but it appears to have been discontinued.[2]
See also[edit]
- NK1 receptor antagonist
- Maropitant (tert-butyl instead of isopropyl)
References[edit]
- ^ a b "International Nonproprietary Names for Pharmaceutical Substances (INN). Recommended International Nonproprietary Names: List 44" (PDF). World Health Organization. pp. 194–5. Retrieved 17 November 2016.
- ^ a b Evangelista S (October 2001). "Eziopitant. Pfizer". Current Opinion in Investigational Drugs. 2 (10): 1441–3. PMID 11890362.
- ^ Diemunsch P, Grélot L (September 2000). "Potential of substance P antagonists as antiemetics". Drugs. 60 (3): 533–46. doi:10.2165/00003495-200060030-00002. PMID 11030465. S2CID 25136161.
- ^ Giardina GA, Gagliardi S, Martinelli M (August 2003). "Antagonists at the neurokinin receptors--recent patent literature". IDrugs: The Investigational Drugs Journal. 6 (8): 758–72. PMID 12917772.
- ^ Tsuchiya M, Fujiwara Y, Kanai Y, et al. (November 2002). "Anti-emetic activity of the novel nonpeptide tachykinin NK1 receptor antagonist ezlopitant (CJ-11,974) against acute and delayed cisplatin-induced emesis in the ferret". Pharmacology. 66 (3): 144–52. doi:10.1159/000063796. PMID 12372904. S2CID 37936941.
- ^ Tsuchiya M, Sakakibara A, Yamamoto M (January 2005). "A tachykinin NK1 receptor antagonist attenuates the 4 beta-phorbol-12-myristate-13-acetate-induced nociceptive behaviour in the rat". European Journal of Pharmacology. 507 (1–3): 29–34. doi:10.1016/j.ejphar.2004.11.028. PMID 15659291.