 | |
| Clinical data | |
|---|---|
| Trade names | Ovulen, Demulen, others |
| Other names | Ethynodiol diacetate; Norethindrol diacetate; 3β-Hydroxynorethisterone 3β,17β-diacetate;[1] 17α-Ethynylestr-4-ene-3β,17β-diyl diacetate; CB-8080; SC-11800 |
| License data |
|
| Pregnancy category |
|
| Routes of administration | By mouth |
| Drug class | Progestogen; Progestin; Progestogen ester |
| ATC code | |
| Legal status | |
| Legal status | |
| Identifiers | |
| |
| CAS Number | |
| PubChem CID | |
| DrugBank | |
| ChemSpider | |
| UNII | |
| KEGG | |
| ChEBI | |
| ChEMBL | |
| CompTox Dashboard (EPA) | |
| ECHA InfoCard | 100.005.496 |
| Chemical and physical data | |
| Formula | C24H32O4 |
| Molar mass | 384.516 g·mol−1 |
| 3D model (JSmol) | |
| |
| |
Etynodiol diacetate, or ethynodiol diacetate, sold under the brand name Ovulen among others, is a progestin medication which is used in birth control pills.[4][5][6] The medication is available only in combination with an estrogen.[7] It is taken by mouth.[8]
Etynodiol diacetate is a progestin, or a synthetic progestogen, and hence is an agonist of the progesterone receptor, the biological target of progestogens like progesterone.[9][10] It has weak androgenic and estrogenic activity and no other important hormonal activity.[11][12][13] The medication is a prodrug of norethisterone in the body, with etynodiol occurring as an intermediate.[9][10][14]
Etynodiol, a related compound, was discovered in 1954, and etynodiol diacetate was introduced for medical use in 1965.[15][16] The combination ethynodiol with mestranol (Ovulen) was approved for medical use in the United States in 1966.[17] The combination ethinylestradiol with ethynodiol (Demulen) was approved for medical use in the United States in 1970.[18]
In 2021, the combination with ethinylestradiol was the 276th most commonly prescribed medication in the United States, with more than 800,000 prescriptions.[19][20]
Medical uses[edit]
Etynodiol diacetate is used in combination with an estrogen such as ethinylestradiol or mestranol in combined oral contraceptives for women for the prevention of pregnancy.[8]
Side effects[edit]
Pharmacology[edit]

Etynodiol diacetate is virtually inactive in terms of affinity for the progesterone and androgen receptors and acts as a rapidly converted prodrug of norethisterone, with etynodiol occurring as an intermediate.[9][10][14] Upon oral administration and during first-pass metabolism in the liver, etynodiol diacetate is rapidly converted by esterases into etynodiol,[14] which is followed by oxygenation of the C3 hydroxyl group to produce norethisterone.[10] In addition to its progestogenic activity, etynodiol diacetate has weak androgenic activity,[11][12] and, unlike most progestins but similarly to norethisterone and noretynodrel,[21] also has some estrogenic activity.[12][13]
The pharmacokinetics of etynodiol diacetate have been reviewed.[22]
| Compound | Typea | PR | AR | ER | GR | MR | SHBG | CBG |
|---|---|---|---|---|---|---|---|---|
| Norethisterone | – | 67–75 | 15 | 0 | 0–1 | 0–3 | 16 | 0 |
| 5α-Dihydronorethisterone | Metabolite | 25 | 27 | 0 | 0 | ? | ? | ? |
| 3α,5α-Tetrahydronorethisterone | Metabolite | 1 | 0 | 0–1 | 0 | ? | ? | ? |
| 3α,5β-Tetrahydronorethisterone | Metabolite | ? | 0 | 0 | ? | ? | ? | ? |
| 3β,5α-Tetrahydronorethisterone | Metabolite | 1 | 0 | 0–8 | 0 | ? | ? | ? |
| Ethinylestradiol | Metabolite | 15–25 | 1–3 | 112 | 1–3 | 0 | 0.18 | 0 |
| Norethisterone acetate | Prodrug | 20 | 5 | 1 | 0 | 0 | ? | ? |
| Norethisterone enanthate | Prodrug | ? | ? | ? | ? | ? | ? | ? |
| Noretynodrel | Prodrug | 6 | 0 | 2 | 0 | 0 | 0 | 0 |
| Etynodiol | Prodrug | 1 | 0 | 11–18 | 0 | ? | ? | ? |
| Etynodiol diacetate | Prodrug | 1 | 0 | 0 | 0 | 0 | ? | ? |
| Lynestrenol | Prodrug | 1 | 1 | 3 | 0 | 0 | ? | ? |
| Notes: Values are percentages (%). Reference ligands (100%) were promegestone for the PR, metribolone for the AR, estradiol for the ER, dexamethasone for the GR, aldosterone for the MR, dihydrotestosterone for SHBG, and cortisol for CBG. Footnotes: a = Active or inactive metabolite, prodrug, or neither of norethisterone. Sources: See template. | ||||||||
Chemistry[edit]
Etynodiol diacetate, also known as 3β-hydroxy-17α-ethynyl-19-nortestosterone 3β,17β-diaceate, 3β-hydroxynorethisterone 3β,17β-diacetate, or 17α-ethynylestr-4-ene-3β,17β-diol 3β,17β-diacetate, is a synthetic estrane steroid and a derivative of testosterone.[1][5][6] It is specifically a derivative of 19-nortestosterone and 17α-ethynyltestosterone, or of norethisterone (17α-ethynyl-19-nortestosterone), in which the C3 ketone group has been dehydrogenated into a C3β hydroxyl group and acetate esters have been attached at the C3β and C17β positions.[5][6] Etynodiol diacetate is the 3β,17β-diacetate ester of etynodiol (17α-ethynylestr-4-ene-3β,17β-diol).[5][6]
Synthesis[edit]
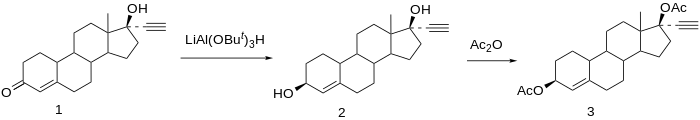
Chemical syntheses of etynodiol diacetate have been published.[22]
Reduction of norethisterone (1) affords the 3,17-diol. The 3β-hydroxy compound is the desired product; since reactions at C3 do not show nearly the stereoselectivity as those at C17 by virtue of the relative lack of stereo-directing proximate substituents, the formation of the desired isomer is engendered by use of a bulky reducing agent, lithium tri-tert-butoxyaluminum hydride. Acetylation of the 3β,17β-diol affords etynodiol diacetate (3).[23]
History[edit]
Etynodiol was first synthesized in 1954, via reduction of norethisterone, and etynodiol diacetate was introduced for medical use in 1965.[15][16]
Society and culture[edit]
Generic names[edit]
Etynodiol diacetate is the generic name of the drug (the INN of its free alcohol form is etynodiol), while ethynodiol diacetate is its USAN, BAN, and JAN.[5][6][7] It is also known by its former developmental code names CB-8080 and SC-11800.[5][6][7]
Brand names[edit]
Etynodiol diacetate is or has been marketed under brand names including Conova, Continuin, Demulen,[18][25] Femulen, Kelnor,[3][25] Lo-Malmorede,[26] Luteonorm, Luto-Metrodiol, Malmorede,[27] Metrodiol, Ovulen,[17][25] Soluna, Zovia,[2] and others.[5][6][7]
Availability[edit]
Etynodiol diacetate is marketed in only a few countries, including the United States, Canada, Argentina, and Oman.[7]
References[edit]
- ^ a b Schindler AE, Campagnoli C, Druckmann R, Huber J, Pasqualini JR, Schweppe KW, et al. (December 2003). "Classification and pharmacology of progestins". Maturitas. 46 (Suppl 1): S7–S16. doi:10.1016/j.maturitas.2003.09.014. PMID 14670641.
- ^ a b "Zovia 1/35- ethynodiol diacetate and ethinyl estradiol tablets kit". Archived from the original on 28 September 2022. Retrieved 20 January 2024.
- ^ a b "Kelnor 1/35- ethynodiol diacetate and ethinyl estradiol kit". Archived from the original on 29 March 2023. Retrieved 20 January 2024.
- ^ Shoupe D, Haseltine FP (6 December 2012). Contraception. Springer Science & Business Media. pp. 21–. ISBN 978-1-4612-2730-4.
- ^ a b c d e f g Elks J (14 November 2014). The Dictionary of Drugs: Chemical Data: Chemical Data, Structures and Bibliographies. Springer. pp. 522–. ISBN 978-1-4757-2085-3.
- ^ a b c d e f g Index Nominum 2000: International Drug Directory. Taylor & Francis US. 2000. p. 422. ISBN 978-3-88763-075-1. Retrieved 30 May 2012.
- ^ a b c d e "Etynodiol". Drugs.com. Archived from the original on 5 February 2018. Retrieved 4 February 2018.
- ^ a b Blum RW (22 October 2013). Adolescent Health Care: Clinical Issues. Elsevier Science. pp. 216–. ISBN 978-1-4832-7738-7.
- ^ a b c Hammerstein J (December 1990). "Prodrugs: advantage or disadvantage?". American Journal of Obstetrics and Gynecology. 163 (6 Pt 2): 2198–2203. doi:10.1016/0002-9378(90)90561-K. PMID 2256526.
- ^ a b c d IARC Working Group on the Evaluation of Carcinogenic Risks to Humans, World Health Organization, International Agency for Research on Cancer (2007). Combined Estrogen-progestogen Contraceptives and Combined Estrogen-progestogen Menopausal Therapy. World Health Organization. pp. 146–. ISBN 978-92-832-1291-1.
- ^ a b Tashjian AH, Armstrong EJ (21 July 2011). Principles of Pharmacology: The Pathophysiologic Basis of Drug Therapy. Lippincott Williams & Wilkins. pp. 523–. ISBN 978-1-4511-1805-6. Archived from the original on 11 January 2023. Retrieved 11 October 2016.
- ^ a b c Becker KL (24 April 2001). Principles and Practice of Endocrinology and Metabolism. Lippincott Williams & Wilkins. p. 1004. ISBN 978-0-7817-1750-2. Retrieved 30 May 2012.
- ^ a b Goroll AH, Mulley AG (27 January 2009). Primary Care Medicine: Office Evaluation and Management of the Adult Patient. Lippincott Williams & Wilkins. p. 876. ISBN 978-0-7817-7513-7. Retrieved 30 May 2012.
- ^ a b c Stanczyk FZ (September 2002). "Pharmacokinetics and potency of progestins used for hormone replacement therapy and contraception". Reviews in Endocrine & Metabolic Disorders. 3 (3): 211–224. doi:10.1023/A:1020072325818. PMID 12215716. S2CID 27018468.
- ^ a b Petrow V (1971). "Antifertility agents". Progress in Medicinal Chemistry. 8 (2): 171–229. doi:10.1016/s0079-6468(08)70130-9. ISBN 9780408703147. PMID 4947236.
- ^ a b William Andrew Publishing (22 October 2013). Pharmaceutical Manufacturing Encyclopedia, 3rd Edition. Elsevier. pp. 1516–. ISBN 978-0-8155-1856-3. Archived from the original on 20 January 2024. Retrieved 4 February 2018.
- ^ a b "Ovulen: FDA-Approved Drugs". U.S. Food and Drug Administration (FDA). Archived from the original on 8 December 2022. Retrieved 20 January 2024.
- ^ a b "Demulen: FDA-Approved Drugs". U.S. Food and Drug Administration (FDA). Archived from the original on 10 May 2021. Retrieved 20 January 2024.
- ^ "The Top 300 of 2021". ClinCalc. Archived from the original on 15 January 2024. Retrieved 14 January 2024.
- ^ "Ethinyl Estradiol; Ethynodiol - Drug Usage Statistics". ClinCalc. Archived from the original on 18 January 2024. Retrieved 14 January 2024.
- ^ Runnebaum BC, Rabe T, Kiesel L (6 December 2012). Female Contraception: Update and Trends. Springer Science & Business Media. pp. 36–. ISBN 978-3-642-73790-9.
- ^ a b Konstitution J (27 November 2013). "Eigenschaften der Gestagene". Handbuch der Experimentellen Pharmakologie. Cham: Springer-Verlag. pp. 14–15, 286. ISBN 978-3-642-99941-3.
- ^ a b Klimstra PD, Colton FB (October 1967). "The synthesis of 3beta-hydroxyestr-4-en-17-one and 3beta-hydroxyandrost-4-en-17-one". Steroids. 10 (4): 411–424. doi:10.1016/0039-128X(67)90119-5. PMID 6064262.
- ^ Sondheimer F, Klibansky Y (1959). "Synthesis of 3β-hydroxy analogues of steroidal hormones, a biologically active class of compounds". Tetrahedron. 5: 15–26. doi:10.1016/0040-4020(59)80066-1.
- ^ a b c "Estrogen and Progestin (Oral Contraceptives)". Archived from the original on 18 January 2024. Retrieved 20 January 2024.
- ^ "Lo-Malmorede". Archived from the original on 24 March 2021. Retrieved 20 January 2024.
- ^ "Malmorede". Archived from the original on 1 October 2023. Retrieved 20 January 2024.