 | |
| Clinical data | |
|---|---|
| ATC code |
|
| Identifiers | |
| |
| CAS Number | |
| PubChem CID | |
| IUPHAR/BPS | |
| ChemSpider | |
| UNII | |
| KEGG | |
| ChEMBL | |
| CompTox Dashboard (EPA) | |
| ECHA InfoCard | 100.208.547 |
| Chemical and physical data | |
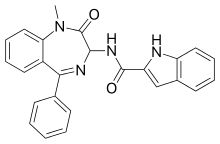
| Formula | C25H20N4O2 |
| Molar mass | 408.461 g·mol−1 |
| 3D model (JSmol) | |
| |
| |
| | |
Devazepide[1] (L-364,718, MK-329) is benzodiazepine drug, but with quite different actions from most benzodiazepines, lacking affinity for GABAA receptors and instead acting as an CCKA receptor antagonist.[2] It increases appetite and accelerates gastric emptying,[3][4] and has been suggested as a potential treatment for a variety of gastrointestinal problems including dyspepsia, gastroparesis and gastric reflux.[5] It is also widely used in scientific research into the CCKA receptor.[6][7]
Synthesis[edit]
Devazepide is synthesised in a similar manner to other benzodiazepines.[8][9]
See also[edit]
References[edit]
- ^ US 4820834, Evans, Ben E.; Freidinger, Roger M. & Bock, Mark G., "Benzodiazepine analogs", published 1989-04-11, assigned to Merck & Co. Inc.
- ^ Hill DR, Woodruff GN (September 1990). "Differentiation of central cholecystokinin receptor binding sites using the non-peptide antagonists MK-329 and L-365,260". Brain Research. 526 (2): 276–83. doi:10.1016/0006-8993(90)91232-6. PMID 2257485. S2CID 23851131.
- ^ Cooper SJ, Dourish CT (December 1990). "Multiple cholecystokinin (CCK) receptors and CCK-monoamine interactions are instrumental in the control of feeding". Physiology & Behavior. 48 (6): 849–57. doi:10.1016/0031-9384(90)90239-z. PMID 1982361. S2CID 24850080.
- ^ Cooper SJ, Dourish CT, Clifton PG (January 1992). "CCK antagonists and CCK-monoamine interactions in the control of satiety". The American Journal of Clinical Nutrition. 55 (1 Suppl): 291S–295S. doi:10.1093/ajcn/55.1.291s. PMID 1728842.
- ^ Scarpignato C, Varga G, Corradi C (1993). "Effect of CCK and its antagonists on gastric emptying". Journal of Physiology, Paris. 87 (5): 291–300. doi:10.1016/0928-4257(93)90035-r. PMID 8298606. S2CID 23725376.
- ^ Weller A (July 2006). "The ontogeny of postingestive inhibitory stimuli: examining the role of CCK". Developmental Psychobiology. 48 (5): 368–79. doi:10.1002/dev.20148. PMID 16770766.
- ^ Savastano DM, Covasa M (October 2007). "Intestinal nutrients elicit satiation through concomitant activation of CCK(1) and 5-HT(3) receptors". Physiology & Behavior. 92 (3): 434–42. doi:10.1016/j.physbeh.2007.04.017. PMID 17531277. S2CID 5566756.
- ^ Evans BE, Rittle KE, Bock MG, DiPardo RM, Freidinger RM, Whitter WL, et al. (December 1988). "Methods for drug discovery: development of potent, selective, orally effective cholecystokinin antagonists". Journal of Medicinal Chemistry. 31 (12): 2235–46. doi:10.1021/jm00120a002. PMID 2848124.
- ^ EP 1492540, Jackson, Karen, "The use of devazepide as analgesic agent", published 2005-01-05, assigned to ML Laboratories plc