| |
| Names | |
|---|---|
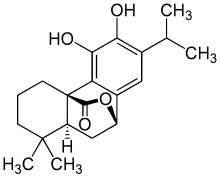
| IUPAC name
11,12-Dihydroxy-7β,20-epoxyabieta-8,11,13-trien-20-one
| |
| Systematic IUPAC name
(4aR,9S,10aS)-5,6-Dihydroxy-1,1-dimethyl-7-(propan-2-yl)-1,3,4,9,10,10a-hexahydro-2H-9,4a-(epoxymethano)phenanthren-12-one | |
| Identifiers | |
3D model (JSmol)
|
|
| ChemSpider | |
PubChem CID
|
|
| UNII | |
CompTox Dashboard (EPA)
|
|
| |
| |
| Properties | |
| C20H26O4 | |
| Molar mass | 330.424 g·mol−1 |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa).
| |
Carnosol is a phenolic diterpene found in the herbs rosemary (Rosmarinus officinalis)[1] and Mountain desert sage (Salvia pachyphylla).[2]
It has been studied in-vitro for anti-cancer effects in various cancer cell types.[3]
See also[edit]
References[edit]
- ^ Ai-Hsiang Lo; Yu-Chih Liang; Shoei-Yn Lin-Shiau; Chi-Tang Ho; Jen-Kun Lin (2002). "Carnosol, an antioxidant in rosemary, suppresses inducible nitric oxide synthase through down-regulating nuclear factor-κB in mouse macrophages". Carcinogenesis. 23 (6): 983–991. doi:10.1093/carcin/23.6.983. PMID 12082020.
- ^ Ivan C. Guerrero; Lucia S. Andres; Leticia G. Leon; Ruben P. Machin; Jose M. Padron; Javier G. Luis; Jose Delgadillo (2006). "Abietane Diterpenoids from Salvia pachyphylla and S. clevelandii with Cytotoxic Activity against Human Cancer Cell Lines". J. Nat. Prod. 69 (12): 1803–1805. doi:10.1021/np060279i. PMID 17190465.
- ^ Johnson JJ (June 2011). "Carnosol: a promising anti-cancer and anti-inflammatory agent". Cancer Lett. 305 (1): 1–7. doi:10.1016/j.canlet.2011.02.005. PMC 3070765. PMID 21382660.