| |
| Names | |
|---|---|
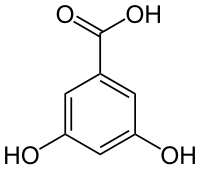
| Preferred IUPAC name
3,5-Dihydroxybenzoic acid | |
| Other names
α-Resorcylic acid
| |
| Identifiers | |
3D model (JSmol)
|
|
| ChEBI | |
| ChEMBL | |
| ChemSpider | |
| ECHA InfoCard | 100.002.482 |
| EC Number |
|
PubChem CID
|
|
| UNII | |
CompTox Dashboard (EPA)
|
|
| |
| |
| Properties | |
| C7H6O4 | |
| Molar mass | 154.121 g·mol−1 |
| Melting point | 235.3 °C (455.5 °F; 508.4 K)[2] |
| Acidity (pKa) | 4.04[1] |
| Hazards | |
| GHS labelling: | |

| |
| Warning | |
| H315, H319, H335 | |
| P261, P264, P271, P280, P302+P352, P304+P340, P305+P351+P338, P312, P321, P332+P313, P337+P313, P362, P403+P233, P405, P501 | |
| Related compounds | |
Related compounds
|
Gallic acid; 4-Hydroxybenzoic acid; Phloroglucinol |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa).
| |
3,5-Dihydroxybenzoic acid (α-resorcylic acid) is a dihydroxybenzoic acid. It is a colorless solid.
Preparation and occurrence[edit]
It is prepared by disulfonation of benzoic acid followed by hydrolysis of the disulfonate.[3]
It is a metabolite of alkylresorcinols, first identified in human urine[4] and can be quantified in urine[5] and plasma,[6] and may be an alternative, equivalent biomarker of whole grain wheat intake.[7]
References[edit]
- ^ Haynes, p. 5.91
- ^ Haynes, p. 3.190
- ^ Weston, Arthur W.; Suter, C. M. (1941). "3,5-Dihydroxybenzoic Acid". Org. Synth. 21: 27. doi:10.15227/orgsyn.021.0027.
- ^ Ross, A. B.; Åman, P.; Kamal-Eldin, A. (2004). "Identification of cereal alkylresorcinol metabolites in human urine—potential biomarkers of wholegrain wheat and rye intake". Journal of Chromatography B. 809 (1): 125–130. doi:10.1016/j.jchromb.2004.06.015. PMID 15282102.
- ^ Koskela, A.; Linko-Parvinen, A. -M.; Hiisivuori, P.; Samaletdin, A.; Kamal-Eldin, A.; Tikkanen, M. J.; Adlercreutz, H. (2007). "Quantification of Alkylresorcinol Metabolites in Urine by HPLC with Coulometric Electrode Array Detection". Clinical Chemistry. 53 (7): 1380–1383. doi:10.1373/clinchem.2006.084764. PMID 17495018.
- ^ Koskela, A.; Samaletdin, A.; Aubertin-Leheudre, M. N.; Adlercreutz, H. (2008). "Quantification of Alkylresorcinol Metabolites in Plasma by High-Performance Liquid Chromatography with Coulometric Electrode Array Detection". Journal of Agricultural and Food Chemistry. 56 (17): 7678–7681. CiteSeerX 10.1.1.533.1473. doi:10.1021/jf801252s. PMID 18690683.
- ^ Aubertin-Leheudre, M.; Koskela, A.; Marjamaa, A.; Adlercreutz, H. (2008). "Plasma Alkylresorcinols and Urinary Alkylresorcinol Metabolites as Biomarkers of Cereal Fiber Intake in Finnish Women". Cancer Epidemiology, Biomarkers & Prevention. 17 (9): 2244–2248. doi:10.1158/1055-9965.EPI-08-0215. PMID 18768490.
Cited sources[edit]
- Haynes, William M., ed. (2016). CRC Handbook of Chemistry and Physics (97th ed.). CRC Press. ISBN 9781498754293.