| Aromatase inhibitor | |
|---|---|
| Drug class | |
 Anastrozole, a non steroidal aromatase inhibitor and a widely used drug in the treatment of breast cancer. | |
| Class identifiers | |
| Synonyms | Estrogen synthesis inhibitors; Estrogen synthase inhibitors; Estrogen blockers |
| Use | Breast cancer, infertility, precocious puberty, medical abortion, gynecomastia, endometriosis, short stature, others |
| ATC code | L02BG |
| Biological target | Aromatase |
| Chemical class | Steroidal; Nonsteroidal |
| Legal status | |
| In Wikidata | |
Aromatase inhibitors (AIs) are a class of drugs used in the treatment of breast cancer in postmenopausal women and in men,[1][2] and gynecomastia in men. They may also be used off-label to reduce estrogen conversion when supplementing testosterone exogenously. They may also be used for chemoprevention in women at high risk for breast cancer.
Aromatase is the enzyme that catalyzes a key aromatization step in the synthesis of estrogen. It converts the enone ring of androgen precursors such as testosterone, to a phenol, completing the synthesis of estrogen. As such, AIs are estrogen synthesis inhibitors. Because hormone-positive breast and ovarian cancers are dependent on estrogen for growth, AIs are taken to either block the production of estrogen or block the action of estrogen on receptors.
Medical uses[edit]
Cancer[edit]
In contrast to premenopausal women, in whom most of the estrogen is produced in the ovaries, in postmenopausal women estrogen is mainly produced in peripheral tissues of the body. Because some breast cancers respond to estrogen, lowering estrogen production at the site of the cancer (i.e. the adipose tissue of the breast) with aromatase inhibitors has been proven to be an effective treatment for hormone-sensitive breast cancer in postmenopausal women.[3] Aromatase inhibitors are generally not used to treat breast cancer in premenopausal women because, prior to menopause, the decrease in estrogen activates the hypothalamus and pituitary axis to increase gonadotropin secretion, which in turn stimulates the ovary to increase androgen production. The heightened gonadotropin levels also upregulate the aromatase promoter, increasing aromatase production in the setting of increased androgen substrate. This would counteract the effect of the aromatase inhibitor in premenopausal women, as total estrogen would increase.
Ongoing areas of clinical research include optimizing adjuvant hormonal therapy in postmenopausal women with breast cancer. Tamoxifen (a SERM) traditionally was the drug treatment of choice, but the ATAC trial (Arimidex, Tamoxifen, Alone or in Combination) showed that in women with localized estrogen receptor-positive breast cancer, women receiving the AI anastrozole had better results than the tamoxifen group.[4] Trials of AIs used as adjuvant therapy, when given to prevent relapse after surgery for breast cancer, show that they are associated with a better disease-free survival than tamoxifen, but few conventionally-analyzed clinicals trials have shown that AIs have an overall survival advantage compared with tamoxifen, and there is no good evidence they are better tolerated.[5]
Gynecomastia[edit]
Aromatase inhibitors have been approved for the treatment of gynecomastia in children and adolescents.[6]
Ovulation induction[edit]
Ovarian stimulation with the aromatase inhibitor letrozole has been proposed for ovulation induction in order to treat unexplained female infertility. In a multi-center study funded by the National Institute of Child Health and Development, ovarian stimulation with letrozole resulted in a significantly lower frequency of multiple gestation (i.e., twins or triplets) but also a lower frequency of live birth, as compared with gonadotropin but not with clomiphene.[7]
Side effects[edit]
In women, side effects include an increased risk for developing osteoporosis and joint disorders such as arthritis, arthrosis, and joint pain. Men do not appear to exhibit the same adverse effects on bone health.[8] Bisphosphonates are sometimes prescribed to prevent the osteoporosis induced by aromatase inhibitors, but also have another serious side effect, osteonecrosis of the jaw. As statins have a bone strengthening effect, combining a statin with an aromatase inhibitor could help prevent fractures and suspected cardiovascular risks, without potential of causing osteonecrosis of the jaw.[9] The more common adverse events associated with the use of aromatase inhibitors include decreased rate of bone maturation and growth, infertility, aggressive behavior, adrenal insufficiency, kidney failure, hair loss,[10][11] and liver dysfunction. Patients with liver, kidney or adrenal abnormalities are at a higher risk of developing adverse events.[12]
Mechanism of action[edit]
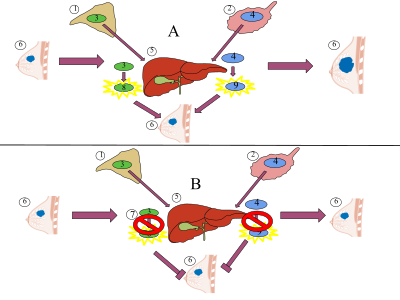
Aromatase inhibitors work by inhibiting the action of the enzyme aromatase, which converts androgens into estrogens by a process called aromatization. As breast tissue is stimulated by estrogens, decreasing their production is a way of suppressing recurrence of the breast tumor tissue. The main source of estrogen is the ovaries in premenopausal women, while in post-menopausal women most of the body's estrogen is produced in peripheral tissues (outside the CNS), and also a few CNS sites in various regions within the brain. Estrogen is produced and acts locally in these tissues, but any circulating estrogen, which exerts systemic estrogenic effects in men and women, is the result of estrogen escaping local metabolism and spreading to the circulatory system.[13]
| Generation | Medication | Dosage | % inhibitiona | Classb | IC50c |
|---|---|---|---|---|---|
| First | Testolactone | 250 mg 4x/day p.o. | ? | Type I | ? |
| 100 mg 3x/week i.m. | ? | ||||
| Rogletimide | 200 mg 2x/day p.o. 400 mg 2x/day p.o. 800 mg 2x/day p.o. |
50.6% 63.5% 73.8% |
Type II | ? | |
| Aminoglutethimide | 250 mg mg 4x/day p.o. | 90.6% | Type II | 4,500 nM | |
| Second | Formestane | 125 mg 1x/day p.o. 125 mg 2x/day p.o. 250 mg 1x/day p.o. |
72.3% 70.0% 57.3% |
Type I | 30 nM |
| 250 mg 1x/2 weeks i.m. 500 mg 1x/2 weeks i.m. 500 mg 1x/1 week i.m. |
84.8% 91.9% 92.5% | ||||
| Fadrozole | 1 mg 1x/day p.o. 2 mg 2x/day p.o. |
82.4% 92.6% |
Type II | ? | |
| Third | Exemestane | 25 mg 1x/day p.o. | 97.9% | Type I | 15 nM |
| Anastrozole | 1 mg 1x/day p.o. 10 mg 1x/day p.o. |
96.7–97.3% 98.1% |
Type II | 10 nM | |
| Letrozole | 0.5 mg 1x/day p.o. 2.5 mg 1x/day p.o. |
98.4% 98.9%–>99.1% |
Type II | 2.5 nM | |
| Footnotes: a = In postmenopausal women. b = Type I: Steroidal, irreversible (substrate-binding site). Type II: Nonsteroidal, reversible (binding to and interference with the cytochrome P450 heme moiety). c = In breast cancer homogenates. Sources: See template. | |||||
Types[edit]
There are two types of aromatase inhibitors approved to treat breast cancer:[14]
- Irreversible steroidal inhibitors, such as exemestane (Aromasin), forms a permanent and deactivating bond with the aromatase enzyme.
- Nonsteroidal inhibitors, such as the triazoles anastrozole (Arimidex) and letrozole (Femara), inhibit the synthesis of estrogen via reversible competition.
Members[edit]

Aromatase inhibitors (AIs) include:
Non-selective[edit]
- Aminoglutethimide (Elipten, Cytadren, Orimeten)
- Testolactone (Teslac)
Selective[edit]
- Anastrozole (Arimidex)
- Letrozole (Femara)
- Exemestane (Aromasin)
- Vorozole (R-76713; Rivizor)
- Formestane (Lentaron)
- Fadrozole (Afema)
Unknown[edit]
- 1,4,6-Androstatrien-3,17-dione (ATD)
- 4-Androstene-3,6,17-trione ("6-OXO")
In addition to pharmaceutical AIs, some natural elements have aromatase inhibiting effects, such as damiana leaves.
History[edit]
The development of aromatase inhibitors was first pioneered by the work of British pharmacologist Angela Brodie at the University of Maryland School of Medicine, first demonstrating efficacy of Formestane in clinical trials in 1982.[15] The drug was first marketed in 1994.[16]
Investigations and research has been undertaken to study the use of aromatase inhibitors to stimulate ovulation, and also to suppress estrogen production.[17] Aromatase inhibitors have been shown to reverse age-related declines in testosterone, including primary hypogonadism.[18] Extracts of certain mushrooms have been shown to inhibit aromatase when evaluated by enzyme assays, with white mushroom having shown the greatest ability to inhibit the enzyme.[19][20] AIs have also been used experimentally in the treatment of adolescents with delayed puberty.[21]
Research[edit]
Research suggests the common table mushroom has anti-aromatase[22] properties and therefore possible anti-estrogen activity. In 2009, a case-control study of the eating habits of 2,018 women in southeast China revealed that women who consumed greater than 10 grams of fresh mushrooms or greater than 4 grams of dried mushrooms per day had an approximately 50% lower incidence of breast cancer. Chinese women who consumed mushrooms and green tea had a 90% lower incidence of breast cancer.[23] However the study was relatively small (2,018 patients participating) and limited to Chinese women of southeast China.
The extract from the herb damiana (Turnera diffusa) has been found to suppress aromatase activity, including the isolated compounds pinocembrin and acacetin.[24][better source needed][25][better source needed]
Natural aromatase inhibitors[edit]
| Species Name | Common Name | Family | Type |
|---|---|---|---|
| Aesculus glabra | Ohio buckeye | Hippocastanaceae | Plant |
| Agaricus bisporus | Baby button mushroom | Agaricaceae | Fungus |
| Allium sp. | White onions | Liliaceae | Plant |
| Alpinia purpurata | Red ginger | Zingerberaceae | Plant |
| Brassica oleracea | Cauliflower | Brassicaceae | Plant |
See also[edit]
References[edit]
- ^ Hassett, Michael J.; Somerfield, Mark R.; Giordano, Sharon H. (2020). "Management of Male Breast Cancer: ASCO Guideline Summary". JCO Oncology Practice. 16 (8): e839–e843. doi:10.1200/JOP.19.00792. PMID 32091951. S2CID 211475185.
- ^ "Hormone Therapy for Breast Cancer in Men".
- ^ Howell A, Cuzick J, Baum M, Buzdar A, Dowsett M, Forbes JF, Hoctin-Boes G, Houghton J, Locker GY, Tobias JS (2005). "Results of the ATAC (Arimidex, Tamoxifen, Alone or in Combination) trial after completion of 5 years' adjuvant treatment for breast cancer". Lancet. 365 (9453): 60–2. doi:10.1016/S0140-6736(04)17666-6. PMID 15639680. S2CID 8350282.
- ^ Howell A, Cuzick J, Baum M, Buzdar A, Dowsett M, Forbes JF, et al. (2005). "Results of the ATAC (Arimidex, Tamoxifen, Alone or in Combination) trial after completion of 5 years' adjuvant treatment for breast cancer". Lancet. 365 (9453): 60–2. doi:10.1016/S0140-6736(04)17666-6. PMID 15639680. S2CID 8350282. [non-primary source needed]
- ^ Seruga B, Tannock IF (2009). "Up-front use of aromatase inhibitors as adjuvant therapy for breast cancer: the emperor has no clothes". J. Clin. Oncol. 27 (6): 840–2. CiteSeerX 10.1.1.617.8757. doi:10.1200/JCO.2008.19.5594. PMID 19139426.
- ^ Shulman, DI; Francis, GL; Palmert, MR; Eugster, EA; Lawson Wilkins Pediatric Endocrine Society Drug and Therapeutics Committee (April 2008). "Use of aromatase inhibitors in children and adolescents with disorders of growth and adolescent development". Pediatrics. 121 (4): e975–983. doi:10.1542/peds.2007-2081. PMID 18381525. S2CID 39852740.
- ^ Diamond MP, Legro RS, Coutifaris R, et al. (2015). "Letrozole, Gonadotropin, or Clomiphene for Unexplained Infertility". N Engl J Med. 373 (13): 1230–1240. doi:10.1056/NEJMoa1414827. PMC 4739644. PMID 26398071.
- ^ Tan, RB; et al. (19 October 2015). "Clinical Use of Aromatase Inhibitors in Adult Males". Sexual Medicine Reviews. 2 (2): 79–90. doi:10.1002/smrj.23. PMID 27784593.
- ^ Ewer MS, Glück S (2009). "A woman's heart: the impact of adjuvant endocrine therapy on cardiovascular health". Cancer. 115 (9): 1813–26. doi:10.1002/cncr.24219. PMID 19235248. S2CID 25842353.
- ^ Simpson, Dene; Curran, Monique P.; Perry, Caroline M. (2004-01-01). "Letrozole: a review of its use in postmenopausal women with breast cancer". Drugs. 64 (11): 1213–1230. doi:10.2165/00003495-200464110-00005. ISSN 0012-6667. PMID 15161328. S2CID 195689537.
- ^ Rossi, A.; Iorio, A.; Scali, E.; Fortuna, M. C.; Mari, E.; Maxia, C.; Gerardi, M.; Framarino, M.; Carlesimo, M. (2013-06-01). "Aromatase inhibitors induce 'male pattern hair loss' in women?". Annals of Oncology. 24 (6): 1710–1711. doi:10.1093/annonc/mdt170. ISSN 0923-7534. PMID 23696617.
- ^ "Aromatase Inhibitors in Products Marketed as Dietary Supplements: Recall" (Press release). FDA. September 20, 2010. Retrieved August 9, 2012.
- ^ Simpson ER (2003). "Sources of estrogen and their importance". J. Steroid Biochem. Mol. Biol. 86 (3–5): 225–30. doi:10.1016/S0960-0760(03)00360-1. PMID 14623515. S2CID 11210435.
- ^ Mokbel K (2002). "The evolving role of aromatase inhibitors in breast cancer". Int. J. Clin. Oncol. 7 (5): 279–83. doi:10.1007/s101470200040. PMID 12402060. S2CID 2862849.
- ^ "Angela Hartley Brodie, PhD". University of Maryland Medical Centre. Archived from the original on 1 February 2016. Retrieved 22 January 2016.
- ^ Grohol, John M. (21 February 2009). "Robert A. Weinberg and Angela M. Hartley Brodie awarded 2006 Landon-AACR Prizes for Cancer Research". PsycheCentral. Archived from the original on 11 August 2017. Retrieved 23 January 2016.
- ^ Attar E, Bulun SE (2006). "Aromatase inhibitors: the next generation of therapeutics for endometriosis?". Fertil. Steril. 85 (5): 1307–18. doi:10.1016/j.fertnstert.2005.09.064. PMID 16647373.
- ^ Leder BZ, Rohrer JL, Rubin SD, Gallo J, Longcope C (2004). "Effects of aromatase inhibition in elderly men with low or borderline-low serum testosterone levels". J. Clin. Endocrinol. Metab. 89 (3): 1174–80. doi:10.1210/jc.2003-031467. PMID 15001605.
- ^ Grube BJ, Eng ET, Kao YC, Kwon A, Chen S (2001). "White button mushroom phytochemicals inhibit aromatase activity and breast cancer cell proliferation". J. Nutr. 131 (12): 3288–93. doi:10.1093/jn/131.12.3288. PMID 11739882.
- ^ Chen S, Oh SR, Phung S, Hur G, Ye JJ, Kwok SL, Shrode GE, Belury M, Adams LS, Williams D (2006). "Anti-aromatase activity of phytochemicals in white button mushrooms (Agaricus bisporus)". Cancer Res. 66 (24): 12026–34. doi:10.1158/0008-5472.CAN-06-2206. PMID 17178902.
- ^ Hero M, Wickman S, Dunkel L (2006). "Treatment with the aromatase inhibitor letrozole during adolescence increases near-final height in boys with constitutional delay of puberty". Clin. Endocrinol. 64 (5): 510–3. doi:10.1111/j.1365-2265.2006.02499.x. PMID 16649968. S2CID 41008940.
- ^ Chen S, Kao YC, Laughton CA (1997). "Binding characteristics of aromatase inhibitors and phytoestrogens to human aromatase". J. Steroid Biochem. Mol. Biol. 61 (3–6): 107–15. doi:10.1016/S0960-0760(97)80001-5. PMID 9365179. S2CID 28593509.
- ^ Zhang M, Huang J, Xie X, Holman CD (March 2009). "Dietary intakes of mushrooms and green tea combine to reduce the risk of breast cancer in Chinese women". Int. J. Cancer. 124 (6): 1404–8. doi:10.1002/ijc.24047. PMID 19048616. S2CID 22781113.
- ^ Zhao J, Dasmahapatra AK, Khan SI, Khan IA (2008). "Anti-aromatase activity of the constituents from damiana (Turnera diffusa)". Journal of Ethnopharmacology. 120 (3): 387–393. doi:10.1016/j.jep.2008.09.016. PMID 18948180.
- ^ Szewczyka K, Zidorn C (2014). "Ethnobotany, phytochemistry, and bioactivity of the genus Turnera (Passifloraceae) with a focus on damiana—Turnera diffusa". Journal of Ethnopharmacology. 152 (3): 424–443. doi:10.1016/j.jep.2014.01.019. PMID 24468305.
- ^ Balunas, M. J.; Su, B.; Brueggemeier, R. W.; Kinghorn, A. D. (2008). "Natural products as aromatase inhibitors". Anti-Cancer Agents in Medicinal Chemistry. 8 (6): 646–682. doi:10.2174/187152008785133092. PMC 3074486. PMID 18690828.
External links[edit]
 Media related to Aromatase inhibitors at Wikimedia Commons
Media related to Aromatase inhibitors at Wikimedia Commons