 | |
| Clinical data | |
|---|---|
| Other names | Allylnorpethidine, WIN-7681 |
| ATC code |
|
| Identifiers | |
| |
| CAS Number | |
| PubChem CID | |
| ChemSpider | |
| CompTox Dashboard (EPA) | |
| Chemical and physical data | |
| Formula | C17H23NO2 |
| Molar mass | 273.376 g·mol−1 |
| 3D model (JSmol) | |
| |
| |
| | |
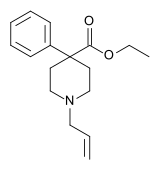
Allylnorpethidine (WIN-7681) is a 4-phenylpiperidine derivative that is related to the opioid analgesic drug pethidine (meperidine).
Allylnorpethidine is an analogue of pethidine where the N-methyl group has been replaced by allyl. In many other opioid derivatives, placing an allyl substituent on the nitrogen instead of a methyl will reverse the normal opioid effects, to produce μ-opioid antagonists which among other things reverse the respiratory depression caused by opioid agonists such as morphine. This is not true with allylnorpethidine, as while it does partially reverse the respiratory depression produced by morphine[1] it is actually an active analgesic and has no antagonistic properties[2][3] when administered alone, so is instead a partial agonist.
References[edit]
- ^ Costa PJ, Bonnycastle DD (March 1955). "The effect of levallorphan tartrate, nalorphine HCl and WIN 7681 (1-allyl-4-phenyl-4-carbethoxypiperidine) on respiratory depression and analgesia induced by some active analgetics". The Journal of Pharmacology and Experimental Therapeutics. 113 (3): 310–8. PMID 14368499.
- ^ Casy AF, Parfitt RT (1986). Opioid Analgesics: Chemistry and Receptors. Springer. p. 239. ISBN 0-306-42130-5.
- ^ Stenlake JB (1979). Foundations of Molecular Pharmacology. p. 152. ISBN 0-485-11171-3.(online version)