| |
| |
| Names | |
|---|---|
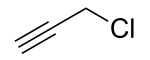
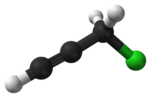
| Preferred IUPAC name
3-Chloroprop-1-yne | |
| Other names
Propargyl chloride, 3-Chloropropyne, 1-Chloro-2-propyne, 2-Propynyl chloride, Gamma-Chloroallylene, UN 2345
| |
| Identifiers | |
3D model (JSmol)
|
|
| ChemSpider | |
| ECHA InfoCard | 100.009.870 |
| EC Number |
|
PubChem CID
|
|
| UNII | |
CompTox Dashboard (EPA)
|
|
| |
| |
| Properties | |
| C3H3Cl | |
| Molar mass | 74.51 g·mol−1 |
| Appearance | colorless liquid |
| Density | 1.0306 g/cm3 |
| Melting point | −78 °C (−108 °F; 195 K) |
| Boiling point | 57 °C (135 °F; 330 K) |
| Insoluble | |
| Hazards | |
| GHS labelling: | |
  
| |
| Danger | |
| H225, H301, H314, H330, H331, H335, H412 | |
| P210, P233, P240, P241, P242, P243, P260, P261, P264, P270, P271, P273, P280, P284, P301+P310, P301+P330+P331, P302+P352, P303+P361+P353, P304+P340, P305+P351+P338, P310, P311, P312, P320, P321, P322, P330, P361, P363, P370+P378, P403+P233, P403+P235, P405, P501 | |
| NFPA 704 (fire diamond) | |
| Flash point | 18 °C (64 °F; 291 K) |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa).
| |
Propargyl chloride is an organic compound with the formula HC2CH2Cl. It is a colorless liquid and a lacrymator. It is an alkylating agent that is used in organic synthesis.[2]
See also[edit]
References[edit]
- ^ *Merck Index, 11th Edition, 7820
- ^ M. Olomucki, J. Y. Le Gall (1987). "Alkoxycarbonylation of Propargyl Chloride: Methyl 4-chloro-2-butynoate". Org. Synth. 65: 47. doi:10.15227/orgsyn.065.0047.
