| |

| |
| Names | |
|---|---|
| IUPAC name
2-Phenylethanol
| |
| Other names
2-Phenylethanol
Phenethyl alcohol Benzyl carbinol β-Hydroxyethylbenzene Benzeneethanol | |
| Identifiers | |
3D model (JSmol)
|
|
| ChEBI | |
| ChEMBL | |
| ChemSpider | |
| DrugBank | |
| ECHA InfoCard | 100.000.415 |
| KEGG | |
PubChem CID
|
|
| UNII | |
CompTox Dashboard (EPA)
|
|
| |
| |
| Properties | |
| C8H10O | |
| Molar mass | 122.167 g·mol−1 |
| Odor | Soft, like roses |
| Density | 1.017 g/cm3 |
| Melting point | −27 °C (−17 °F; 246 K) |
| Boiling point | 219 to 221 °C (426 to 430 °F; 492 to 494 K) |
| log P | 1.36 [2] |
| Hazards | |
| NFPA 704 (fire diamond) | |
| Safety data sheet (SDS) | JT Baker MSDS |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa).
| |
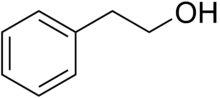
Phenethyl alcohol, or 2-phenylethanol, is an organic compound with the chemical formula C6H5CH2CH2OH. It is a colourless liquid with a pleasant floral odor. It occurs widely in nature, being found in a variety of essential oils. It is slightly soluble in water (2 ml per 100 ml of H2O), but miscible with most organic solvents. The molecule of phenethyl alcohol consists of a phenethyl group (C6H5CH2CH2−) attached to a hydroxyl group (−OH).
Synthesis[edit]
Phenethyl alcohol is prepared commercially via two routes. Most common is the Friedel-Crafts reaction between benzene and ethylene oxide in the presence of aluminium trichloride.
- C6H6 + CH2CH2O + AlCl3 → C6H5CH2CH2OAlCl2 + HCl
The reaction affords the aluminium alkoxide that is subsequently hydrolyzed to the desired product. The main side product is diphenylethane, which can be avoided by use of excess benzene. Hydrogenation of styrene oxide also affords phenethyl alcohol.[3]
Laboratory methods[edit]
Phenethyl alcohol can also be prepared by the reaction between phenylmagnesium bromide and ethylene oxide:
- C6H5MgBr + CH2CH2O → C6H5CH2CH2OMgBr
- C6H5CH2CH2OMgBr + H+ → C6H5CH2CH2OH + MgBr+
Phenethyl alcohol can also be produced by biotransformation from L-phenylalanine using immobilized yeast Saccharomyces cerevisiae.[4] It is also possible to produce phenethyl alcohol by the reduction of phenylacetic acid using sodium borohydride and iodine in THF. [5]
Occurrence and uses[edit]
Phenethyl alcohol is found in extract of rose, carnation, hyacinth, Aleppo pine, orange blossom, ylang-ylang, geranium, neroli, and champaca. It is also an autoantibiotic produced by the fungus Candida albicans.[6]
Fusel alcohols like phenethyl alcohol are grain fermentation byproducts, and therefore trace amounts of phenethyl alcohol are present in many alcoholic beverages.
It is therefore a common ingredient in flavors and perfumery, particularly when the odor of rose is desired.[3] It is used as an additive in cigarettes. It is also used as a preservative in soaps due to its stability in basic conditions. It is of interest due to its antimicrobial properties.
See also[edit]
References[edit]
- ^ Merck Index (11th ed.). p. 7185.
- ^ "Phenylethyl alcohol_msds".
- ^ a b Fahlbusch, Karl-Georg; Hammerschmidt, Franz-Josef; Panten, Johannes; Pickenhagen, Wilhelm; Schatkowski, Dietmar; Bauer, Kurt; Garbe, Dorothea; Surburg, Horst (2003). "Flavors and Fragrances". Ullmann's Encyclopedia of Industrial Chemistry. doi:10.1002/14356007.a11_141. ISBN 978-3-527-30673-2.
- ^ Eshkol N, Sendovski M, Bahalul M, Katz-Ezov T, Kashi Y, Fishman A (2009). "Production of 2-phenylethanol from L-phenylalanine by a stress tolerant Saccharomyces cerevisiae strain". Journal of Applied Microbiology. 106 (2): 534–542. doi:10.1111/j.1365-2672.2008.04023.x. PMID 19200319.
- ^ Kanth JV, Periasamy M (1991). "Selective Reduction of Carboxylic Acids into Alcohols Using NaBH and I2". Journal of Organic Chemistry. 56: 5964–5965. doi:10.1021/jo00020a052.
- ^ Lingappa, BT; Prasad, M; Lingappa, Y; Hunt, DF; Biemann, K (1969). "Phenethyl alcohol and tryptophol: Autoantibiotics produced by the fungus Candida albicans". Science. 163 (3863): 192–4. Bibcode:1969Sci...163..192L. doi:10.1126/science.163.3863.192. PMID 5762768. S2CID 12430791.
