 | |
| Clinical data | |
|---|---|
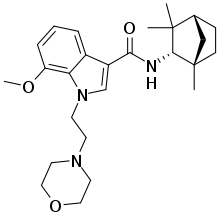
| Other names | N-[(S)-Fenchyl]-1-[2-(morpholin-4-yl)ethyl]-7-methoxyindole-3-carboxamide |
| Legal status | |
| Legal status |
|
| Identifiers | |
| |
| CAS Number |
|
| PubChem CID | |
| ChemSpider | |
| UNII | |
| ChEMBL | |
| Chemical and physical data | |
| Formula | C26H37N3O3 |
| Molar mass | 439.600 g·mol−1 |
| 3D model (JSmol) | |
| |
| |
| (verify) | |
MN-25 (UR-12) is a drug invented by Bristol-Myers Squibb,[1] that acts as a reasonably selective agonist of peripheral cannabinoid receptors.[2] It has moderate affinity for CB2 receptors with a Ki of 11 nM, but 22x lower affinity for the psychoactive CB1 receptors with a Ki of 245 nM. The indole 2-methyl derivative has the ratio of affinities reversed however, with a Ki of 8 nM at CB1 and 29 nM at CB2,[3][4] which contrasts with the usual trend of 2-methyl derivatives having increased selectivity for CB2 (cf. JWH-018 vs JWH-007, JWH-081 vs JWH-098).[5][6]
Chemically, it is closely related to another indole-3-carboxamide synthetic cannabinoid, Org 28611, but with a different cycloalkyl substitution on the carboxamide, and the cyclohexylmethyl group replaced by morpholinylethyl, as in JWH-200 or A-796,260. Early compounds such as these have subsequently led to the development of many related indole-3-carboxamide cannabinoid ligands.[7][8][9][10]
See also[edit]
References[edit]
- ^ WO application 0158869, Leftheris K, Zhao R, Chen BC, Kiener P, Wu H, Pandit CR, Wrobleski S, Chen P, Hynes J, Longphre M, Norris DJ, Spergel S, Tokarski J, "Cannabinoid Receptor Modulators, Their Processes of Preparation, and use of Cannabinoid Receptor Modulators for Treating Respiratory and Non-Respiratory Diseases", published 16 August 2001, assigned to Bristol-Myers Squibb
- ^ Zhao R, Wang B, Wu H, Hynes J, Leftheris K, Balasubramanian B, Barrish JC, Chen BC (20 December 2009). "Improved procedure for the preparation of 7-methoxy-2-methyl-1-(2-morpholinoethyl)-1H-indole-3-carboxylic acid, key intermediate in the synthesis of novel 3-amidoindole and indolopyridone cannabinoid ligands". Arkivoc. 2010 (6): 89–95. doi:10.3998/ark.5550190.0011.610. hdl:2027/spo.5550190.0011.610.
- ^ Hynes J, Leftheris K, Wu H, Pandit C, Chen P, Norris DJ, et al. (September 2002). "C-3 Amido-indole cannabinoid receptor modulators". Bioorganic & Medicinal Chemistry Letters. 12 (17): 2399–402. doi:10.1016/S0960-894X(02)00466-3. PMID 12161142.
- ^ Wrobleski ST, Chen P, Hynes J, Lin S, Norris DJ, Pandit CR, Spergel S, Wu H, Tokarski JS, Chen X, Gillooly KM, Kiener PA, McIntyre KW, Patil-Koota V, Shuster DJ, Turk LA, Yang G, Leftheris K (May 2003). "Rational design and synthesis of an orally active indolopyridone as a novel conformationally constrained cannabinoid ligand possessing antiinflammatory properties". Journal of Medicinal Chemistry. 46 (11): 2110–6. doi:10.1021/jm020329q. PMID 12747783.
- ^ Huffman JW, Padgett LW (2005). "Recent developments in the medicinal chemistry of cannabimimetic indoles, pyrroles and indenes". Current Medicinal Chemistry. 12 (12): 1395–411. doi:10.2174/0929867054020864. PMID 15974991.
- ^ Manera C, Tuccinardi T, Martinelli A (April 2008). "Indoles and related compounds as cannabinoid ligands". Mini Reviews in Medicinal Chemistry. 8 (4): 370–87. doi:10.2174/138955708783955935. PMID 18473928.
- ^ Adam JM, Cairns J, Caulfield W, Cowley P, Cumming I, Easson M, et al. (2010). "Design, synthesis, and structure–activity relationships of indole-3-carboxamides as novel water soluble cannabinoid CB1 receptor agonists". MedChemComm. 1. Royal Society of Chemistry: 54–60. doi:10.1039/c0md00022a.
- ^ Kiyoi T, York M, Francis S, Edwards D, Walker G, Houghton AK, Cottney JE, Baker J, Adam JM (August 2010). "Design, synthesis, and structure-activity relationship study of conformationally constrained analogs of indole-3-carboxamides as novel CB1 cannabinoid receptor agonists". Bioorganic & Medicinal Chemistry Letters. 20 (16): 4918–21. doi:10.1016/j.bmcl.2010.06.067. PMID 20634067.
- ^ Moir EM, Yoshiizumi K, Cairns J, Cowley P, Ferguson M, Jeremiah F, Kiyoi T, Morphy R, Tierney J, Wishart G, York M, Baker J, Cottney JE, Houghton AK, McPhail P, Osprey A, Walker G, Adam JM (December 2010). "Design, synthesis, and structure-activity relationship study of bicyclic piperazine analogs of indole-3-carboxamides as novel cannabinoid CB1 receptor agonists". Bioorganic & Medicinal Chemistry Letters. 20 (24): 7327–30. doi:10.1016/j.bmcl.2010.10.061. PMID 21074434.
- ^ Blaazer AR, Lange JH, van der Neut MA, Mulder A, den Boon FS, Werkman TR, Kruse CG, Wadman WJ (October 2011). "Novel indole and azaindole (pyrrolopyridine) cannabinoid (CB) receptor agonists: design, synthesis, structure-activity relationships, physicochemical properties and biological activity". European Journal of Medicinal Chemistry. 46 (10): 5086–98. doi:10.1016/j.ejmech.2011.08.021. PMID 21885167.
Further reading[edit]
- Hynes J, Leftheris K, Wu H, Pandit C, Chen P, Norris DJ, et al. (September 2002). "C-3 Amido-indole cannabinoid receptor modulators". Bioorganic & Medicinal Chemistry Letters. 12 (17): 2399–402. doi:10.1016/s0960-894x(02)00466-3. PMID 12161142.
- Frost JM, Dart MJ, Tietje KR, Garrison TR, Grayson GK, Daza AV, et al. (January 2010). "Indol-3-ylcycloalkyl ketones: effects of N1 substituted indole side chain variations on CB(2) cannabinoid receptor activity". Journal of Medicinal Chemistry. 53 (1): 295–315. doi:10.1021/jm901214q. PMID 19921781.
- Chin CL, Tovcimak AE, Hradil VP, Seifert TR, Hollingsworth PR, Chandran P, et al. (January 2008). "Differential effects of cannabinoid receptor agonists on regional brain activity using pharmacological MRI". British Journal of Pharmacology. 153 (2): 367–79. doi:10.1038/sj.bjp.0707506. PMC 2219521. PMID 17965748.