 | |
| Clinical data | |
|---|---|
| Other names | Diampromide |
| ATC code |
|
| Legal status | |
| Legal status |
|
| Identifiers | |
| |
| CAS Number | |
| PubChem CID | |
| DrugBank | |
| ChemSpider | |
| UNII | |
| KEGG | |
| ChEMBL | |
| Chemical and physical data | |
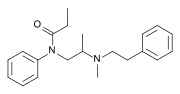
| Formula | C21H28N2O |
| Molar mass | 324.468 g·mol−1 |
| 3D model (JSmol) | |
| |
| |
| (verify) | |
Diampromide is an opioid analgesic from the ampromide family of drugs, related to other drugs such as propiram and phenampromide. It was invented in the 1960s by American Cyanamid,[2] and can be described as a ring-opened analogue of fentanyl.[3]
Diampromide produces similar effects to other opioids, including analgesia, sedation, dizziness and nausea, and is around the same potency as morphine.[4]
Diampromide is in Schedule I of the Controlled Substances Act 1970 of the United States as a Narcotic with ACSCN 9615 with a zero aggregate manufacturing quota as of 2014.[5] It is listed under the Single Convention for the Control of Narcotic Substances 1961 and is controlled in most countries in the same fashion as is morphine.
See also[edit]
References[edit]
- ^ Anvisa (2023-03-31). "RDC Nº 784 - Listas de Substâncias Entorpecentes, Psicotrópicas, Precursoras e Outras sob Controle Especial" [Collegiate Board Resolution No. 784 - Lists of Narcotic, Psychotropic, Precursor, and Other Substances under Special Control] (in Brazilian Portuguese). Diário Oficial da União (published 2023-04-04). Archived from the original on 2023-08-03. Retrieved 2023-08-16.
- ^ US 2944081, "Diphenylalkylenediamines and Methods of Preparation of the Same"
- ^ "Theoretical Study of Acyclic FENTANYL ANALOGS With Analgesic Activity: Diampromide and seco-Fentanyl".
- ^ Ivanovic MD, Micovic IV, Vuckovic S, Prostran M, Todorovic Z, Ivanovic ER, Kiricojevic VD, Djordjevic JB, Dosen-Micovic LJ (2004). "The synthesis and pharmacological evaluation of 2,3-seco-fentanyl analogues". Journal of the Serbian Chemical Society. 69 (11): 955–968. doi:10.2298/JSC0411955I.
- ^ "Final Adjusted Aggregate Production Quotas for Schedule I and II Controlled Substances and Assessment of Annual Needs for the List I Chemicals Ephedrine, Pseudoephedrine, and Phenylpropanolamine for 2014". Drug Enforcement Administration. Archived from the original on 2016-03-04. Retrieved 2016-02-26.