| |
| Names | |
|---|---|
| Preferred IUPAC name
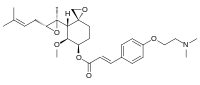
(3R,4S,5S,6R)-5-Methoxy-4-[(2R,3R)-2-methyl-3-(3-methylbut-2-en-1-yl)oxiran-2-yl]-1-oxaspiro[2.5]octan-6-yl (2E)-3-{4-[2-(dimethylamino)ethoxy]phenyl}prop-2-enoate | |
| Other names
CKD-732; ZGN-433
| |
| Identifiers | |
3D model (JSmol)
|
|
| ChemSpider | |
PubChem CID
|
|
| UNII |
|
CompTox Dashboard (EPA)
|
|
| |
| |
| Properties | |
| C29H41NO6 | |
| Molar mass | 499.648 g·mol−1 |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa).
| |
Beloranib is a former drug candidate for the treatment of obesity. It was discovered by CKD Pharmaceuticals and its clinical development was led by Zafgen.[1] Drug development was halted in 2016 after deaths during clinical trials.[2]
Mechanism of action[edit]
Beloranib, an analog of the natural chemical compound fumagillin, is an inhibitor of the enzyme METAP2.[3] It was originally designed as angiogenesis inhibitor for the treatment of cancer.[4] However, once the potential anti-obesity effects of METAP2 inhibition became apparent, the clinical development began to focus on these effects and beloranib has shown positive results in preliminary clinical trials for this indication.[5]
Clinical trials[edit]
A Phase I trial was published in 2013,[6] finding a dose that led to weight loss in obese women in comparison to placebo. Results from a Phase II clinical trial for obesity were promising with clinically meaningful weight loss and improvements in cardiometabolic risk factors in the treated group.[7] Zafgen continued with a Phase III trial for Prader–Willi syndrome.[8]
In December 2015, Zafgen halted the Phase III clinical trial of beloranib for Prader–Willi syndrome after a second patient death in order to determine whether the deaths were treatment-related.[9] After discussions with the Food and Drug Administration indicated that the obstacles to gaining approval were insurmountable, product development for beloranib was ended.[2]
References[edit]
- ^ "News Release: Zafgen Secures $33 Million Series C Financing" (PDF). Zafgen, Inc. July 7, 2011. Archived from the original (PDF) on December 10, 2011.
- ^ a b "Zafgen Halts Development of Beloranib, to Cut Jobs by ~34%". nasdaq.com. July 20, 2016.
- ^ Chun E, Han CK, Yoon JH, Sim TB, Kim YK, Lee KY (March 2005). "Novel inhibitors targeted to methionine aminopeptidase 2 (MetAP2) strongly inhibit the growth of cancers in xenografted nude model". International Journal of Cancer. 114 (1): 124–30. doi:10.1002/ijc.20687. PMID 15523682.
- ^ Kim EJ, Shin WH (February 2005). "General pharmacology of CKD-732, a new anticancer agent: effects on central nervous, cardiovascular, and respiratory system". Biological & Pharmaceutical Bulletin. 28 (2): 217–23. doi:10.1248/bpb.28.217. PMID 15684472.
- ^ "Zafgen Announces Positive Topline Phase 1b Data for ZGN-433 in Obesity". MedNews. Drugs.com. 5 January 2011.
- ^ Hughes TE, Kim DD, Marjason J, Proietto J, Whitehead JP, Vath JE (September 2013). "Ascending dose-controlled trial of beloranib, a novel obesity treatment for safety, tolerability, and weight loss in obese women". Obesity. 21 (9): 1782–8. doi:10.1002/oby.20356. PMID 23512440. S2CID 2352854.
- ^ Kim DD, Krishnarajah J, Lillioja S, de Looze F, Marjason J, Proietto J, et al. (June 2015). "Efficacy and safety of beloranib for weight loss in obese adults: a randomized controlled trial". Diabetes, Obesity & Metabolism. 17 (6): 566–572. doi:10.1111/dom.12457. PMID 25732625. S2CID 205076412.
- ^ "Clinical Trials". Retrieved 2014-11-18.
- ^ "UPDATE 4-Zafgen halts obesity drug trial after second patient death". Reuters. 3 December 2015. Archived from the original on 2015-12-03. Retrieved 2015-12-03.