| |

| |
| Names | |
|---|---|
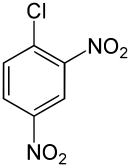
| Preferred IUPAC name
1-Chloro-2,4-dinitrobenzene | |
| Other names
Dinitrochlorobenzene
Chlorodinitrobenzene 2,4-Dinitrochlorobenzene 2,4-Dinitrophenyl chloride 4-Chloro-1,3-dinitrobenzene | |
| Identifiers | |
3D model (JSmol)
|
|
| Abbreviations | CDNB; DNCB |
| ChEBI | |
| ChemSpider | |
| ECHA InfoCard | 100.002.321 |
| EC Number |
|
PubChem CID
|
|
| UNII | |
CompTox Dashboard (EPA)
|
|
| |
| |
| Properties | |
| C6H3ClN2O4 | |
| Molar mass | 202.55 g·mol−1 |
| Appearance | yellow crystals |
| Odor | almond-like |
| Density | 1.6867 g/cm3 |
| Melting point | 54 °C (129 °F; 327 K) |
| Boiling point | 315 °C (599 °F; 588 K) |
| Insoluble[1] | |
| Solubility | soluble in ether, benzene, CS2 |
Refractive index (nD)
|
1.5857 (60 °C) |
| Hazards | |
| NFPA 704 (fire diamond) | |
| Explosive limits | 2–22% |
| Lethal dose or concentration (LD, LC): | |
LD50 (median dose)
|
1.07 g/kg (rat, oral) |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa).
| |
2,4-Dinitrochlorobenzene (DNCB) is an organic compound with the chemical formula (O2N)2C6H3Cl. It is a yellow solid that is soluble in organic solvents. It is an important intermediate for the industrial production of other compounds.[2]
DNCB is produced commercially by the nitration of p-nitrochlorobenzene with a mixture of nitric and sulfuric acids. Other methods afford the compound less efficiently include the chlorination of dinitrobenzene, nitration of o-nitrochlorobenzene and the dinitration of chlorobenzene.[3]
Uses[edit]
By virtue of the two nitro groups, the chloride is susceptible to nucleophilic substitution. In this way, the compound is a precursor to many other compounds.[4][5][6]
Laboratory use[edit]
DNCB is used as a substrate in GST enzyme activity assays.[7] The molecule is conjugated to a single molecule of reduced glutathione which then absorbs at 340 nm. Affinity of CDNB for each class of GST varies and so it is not a good measure of activity for some forms (e.g. GSTT and GSTZ).[citation needed]
Medical use[edit]
DNCB can be used to treat warts with an effective cure rate of 80%.[8] DNCB induces an allergic immune response toward the wart-causing virus.[8]
Safety[edit]
DNCB induces a type IV hypersensitivity reaction in almost all people exposed to it, so it is used medically to assess the T cell activity in patients. This is a useful diagnostic test for immunocompromised patients. It can also be used to treat warts.[9]
DNCB can cause contact dermatitis.[10]
References[edit]
- ^ "1-Chloro-2,4-dinitrobenzene". Sigma-Aldrich. Retrieved 8 September 2014.
- ^ Gerald Booth (2007). "Nitro Compounds, Aromatic". Ullmann's Encyclopedia of Industrial Chemistry. Weinheim: Wiley-VCH. doi:10.1002/14356007.a17_411. ISBN 978-3527306732.
- ^ "Synthesis of 1-chloro-2,4-dinitrobenzene - F. Ullmann, Verlag S. Hirzel Leipzig, 1908" (PDF). Retrieved 19 May 2020.
- ^ J. F. Bunnett, R. M. Conner (1960). "2,4-Dinitroiodobenzene". Organic Syntheses. 40: 34. doi:10.15227/orgsyn.040.0034.
- ^ F. B. Wells, C. F. H. Allen (1935). "2,4-Dinitroaniline". Organic Syntheses. 15: 22. doi:10.15227/orgsyn.015.0022.
- ^ Norman Kharasch, Robert B. Langford (1964). "2,4-Dinitrobenzenesulfenyl Chloride". Organic Syntheses. 44: 47. doi:10.15227/orgsyn.044.0047.
- ^ Habig WH, Pabst MJ, Jakoby WB (1974). "Glutathione S-transferases. The first enzymatic step in mercapturic acid formation". J Biol Chem. 249 (22): 7130–7139. doi:10.1016/S0021-9258(19)42083-8. PMID 4436300.
- ^ a b "Treating Warts". Harvard Health Publications. Harvard Medical School. 21 September 2011.
- ^ "Treating warts". Harvard Medical School. Archived from the original on 2010-11-03. Retrieved April 2, 2010.
- ^ White SI, Friedmann PS, Moss C, Simpson JM (1986). "The effect of altering area of application and dose per unit area on sensitization by DNCB". Br. J. Dermatol. 115 (6): 663–8. doi:10.1111/j.1365-2133.1986.tb06646.x. PMID 3801307. S2CID 21476276.
