| |
| |
| Names | |
|---|---|
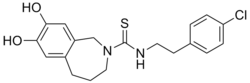
| Preferred IUPAC name
N-[2-(4-Chlorophenyl)ethyl]-7,8-dihydroxy-1,3,4,5-tetrahydro-2H-2-benzazepine-2-carbothioamide | |
Other names
| |
| Identifiers | |
3D model (JSmol)
|
|
| ChEMBL | |
| ChemSpider | |
PubChem CID
|
|
| UNII | |
CompTox Dashboard (EPA)
|
|
| |
| |
| Properties | |
| C19H21ClN2O2S | |
| Molar mass | 376.9 g/mol |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa).
| |
Capsazepine is a synthetic antagonist of capsaicin.[1] It is used as a biochemical tool in the study of TRPV ion channels.
Pharmacology[edit]
Capsazepine blocks the painful sensation of heat caused by capsaicin (the active ingredient of chilli pepper) which activates the TRPV1 ion channel. Capsazepine is therefore considered to be a TRPV1 antagonist. The TRPV1 channel functions as a pain and temperature sensor in mammalians. Capsazepine blocks the activation of TRPV1 channels by other chemicals, but not by other painful stimuli such as heat. Depending on the pharmacological assay, the IC50 is in the nanomolar to low micromolar range. In addition to its effects on TRPV1 channels, it was also shown to activate the noxious chemical sensor TRPA1 channel,[2] inhibit the cold activated TRPM8 channel,[3] voltage-activated calcium channels[4] and nicotinic acetylcholine receptors.[5] It mainly serves as a tool to study the TRPV1 ion channel.[6]
Development[edit]
Capsazepine was discovered by a research group working for Novartis.[1] Its synthesis and chemical properties were published in 1994. It was found by modification of the chemical backbone of capsaicin.[7]
Use in biotechnology[edit]
By incorporation of an azobenzene unit, a photoswitchable version of capsazepine (AC4) was developed in 2013 that allows for optical control of TRPV1 channels with light.[8][9]
See also[edit]
References[edit]
- ^ a b Bevan S, Hothi S, Hughes G, James IF, Rang HP, Shah K, Walpole CS, Yeats JC (October 1992). "Capsazepine: a competitive antagonist of the sensory neurone excitant capsaicin". British Journal of Pharmacology. 107 (2): 544–52. doi:10.1111/j.1476-5381.1992.tb12781.x. PMC 1907893. PMID 1422598.
- ^ Kistner K, Siklosi N, Babes A, Khalil M, Selescu T, Zimmermann K, Wirtz S, Becker C, Neurath MF, Reeh PW, Engel MA (June 2016). "Systemic desensitization through TRPA1 channels by capsazepine and mustard oil - a novel strategy against inflammation and pain". Scientific Reports. 6: 28621. doi:10.1038/srep28621. PMC 4928060. PMID 27356469.
- ^ Behrendt HJ, Germann T, Gillen C, Hatt H, Jostock R (February 2004). "Characterization of the mouse cold-menthol receptor TRPM8 and vanilloid receptor type-1 VR1 using a fluorometric imaging plate reader (FLIPR) assay". British Journal of Pharmacology. 141 (4): 737–45. doi:10.1038/sj.bjp.0705652. PMC 1574235. PMID 14757700.
- ^ Docherty RJ, Yeats JC, Piper AS (August 1997). "Capsazepine block of voltage-activated calcium channels in adult rat dorsal root ganglion neurones in culture". British Journal of Pharmacology. 121 (7): 1461–7. doi:10.1038/sj.bjp.0701272. PMC 1564831. PMID 9257928.
- ^ Liu L, Simon SA (May 1997). "Capsazepine, a vanilloid receptor antagonist, inhibits nicotinic acetylcholine receptors in rat trigeminal ganglia". Neuroscience Letters. 228 (1): 29–32. doi:10.1016/S0304-3940(97)00358-3. PMID 9197280.
- ^ Valenzano KJ, Sun Q (December 2004). "Current perspectives on the therapeutic utility of VR1 antagonists". Current Medicinal Chemistry. 11 (24): 3185–202. doi:10.2174/0929867043363686. PMID 15579007. Archived from the original on 2013-04-14.
- ^ Walpole CS, Bevan S, Bovermann G, Boelsterli JJ, Breckenridge R, Davies JW, Hughes GA, James I, Oberer L, Winter J (June 1994). "The discovery of capsazepine, the first competitive antagonist of the sensory neuron excitants capsaicin and resiniferatoxin". Journal of Medicinal Chemistry. 37 (13): 1942–54. doi:10.1021/jm00039a006. PMID 8027976.
- ^ Stein M, Breit A, Fehrentz T, Gudermann T, Trauner D (September 2013). "Optical control of TRPV1 channels". Angewandte Chemie. 52 (37): 9845–8. doi:10.1002/anie.201302530. PMID 23873837.
- ^ Optical switches: Putting the fire out with light LMU Munich, 07/25/2013