 | |
 | |
| Clinical data | |
|---|---|
| Trade names | Onbrez, Arcapta |
| AHFS/Drugs.com | International Drug Names |
| License data |
|
| Pregnancy category |
|
| Routes of administration | Inhalation |
| ATC code | |
| Legal status | |
| Legal status | |
| Identifiers | |
| |
| CAS Number | |
| PubChem CID | |
| IUPHAR/BPS | |
| ChemSpider | |
| UNII | |
| KEGG | |
| ChEBI | |
| ChEMBL | |
| CompTox Dashboard (EPA) | |
| ECHA InfoCard | 100.218.577 |
| Chemical and physical data | |
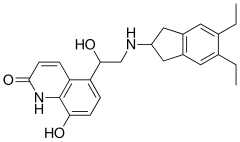
| Formula | C24H28N2O3 |
| Molar mass | 392.499 g·mol−1 |
| 3D model (JSmol) | |
| |
| |
| | |
Indacaterol is an ultra-long-acting beta-adrenoceptor agonist[5] developed by Novartis. It needs to be taken only once a day,[6] unlike the related drugs formoterol and salmeterol. It is licensed only for the treatment of chronic obstructive pulmonary disease (COPD) (long-term data in patients with asthma are thus far lacking). It is delivered as an aerosol formulation through a dry powder inhaler.
Medical uses[edit]
A Cochrane review found benefit in lung function in people with COPD at least as good as that seen with twice-daily long-acting beta2-agonists.[7]
History[edit]
It was approved by the European Medicines Agency (EMA) under the brand name Onbrez Breezhaler on November 30, 2009,[8] and by the United States Food and Drug Administration (FDA), under the brand name Arcapta Neohaler, on July 1, 2011.[9][10] In 2016, Novartis licensed its U.S. commercial rights for Arcapta Neohaler to Sunovion Pharmaceuticals.[11]
References[edit]
- ^ "Arcapta Neohaler (indacaterol) inhalation powder Initial U.S. Approval: 2011". DailyMed. 1 April 2020. Retrieved 14 June 2021.
- ^ "Onbrez Breezhaler EPAR". European Medicines Agency (EMA). 17 September 2018. Retrieved 20 January 2021.
- ^ "Oslif Breezhaler EPAR". European Medicines Agency (EMA). 17 September 2018. Retrieved 20 January 2021.
- ^ "Hirobriz Breezhaler EPAR". European Medicines Agency (EMA). 17 September 2018. Retrieved 20 January 2021.
- ^ Cazzola M, Matera MG, Lötvall J (July 2005). "Ultra long-acting beta 2-agonists in development for asthma and chronic obstructive pulmonary disease". Expert Opin Investig Drugs. 14 (7): 775–83. doi:10.1517/13543784.14.7.775. PMID 16022567. S2CID 11930383.
- ^ Beeh KM, Derom E, Kanniess F, Cameron R, Higgins M, van As A (May 2007). "Indacaterol, a novel inhaled beta2-agonist, provides sustained 24-h bronchodilation in asthma". Eur. Respir. J. 29 (5): 871–8. doi:10.1183/09031936.00060006. PMID 17251236.
- ^ Geake, James B (2015). "Indacaterol, a once-daily beta2-agonist, versus twice-daily beta2-agonists or placebo for chronic obstructive pulmonary disease". Reviews. 1 (3): CD010139. doi:10.1002/14651858.CD010139.pub2. PMC 6464646. PMID 25575340.
- ^ European Public Assessment Report for Onbrez Breezhaler Archived 2010-01-16 at the Wayback Machine
- ^ "FDA approves Arcapta Neohaler to treat chronic obstructive pulmonary disease" (Press release). U.S. Food and Drug Administration. 2011-07-01. Archived from the original on 2011-07-03. Retrieved 2011-07-02.
- ^ "Drug Approval Package: Arcapta Neohaler (indacaterol maleate) NDA #022383". U.S. Food and Drug Administration. 13 August 2013. Retrieved 14 June 2021.
- ^ Faulkner, Sarah (22 December 2016). "Sunovion, Novartis ink licensing deal for inhaled COPD drugs". Drug Delivery Business.