 | |
 | |
| Clinical data | |
|---|---|
| Trade names | nefopam medisol |
| AHFS/Drugs.com | International Drug Names |
| Routes of administration | intramuscular, intravenous |
| ATC code | |
| Legal status | |
| Legal status | |
| Pharmacokinetic data | |
| Bioavailability | Low[1] |
| Protein binding | 70–75% (mean 73%)[1][2] |
| Metabolism | Liver (N-demethylation, others)[1] |
| Metabolites | Desmethylnefopam, others[1] |
| Elimination half-life | Nefopam: 3–8 hours[1] Desmethylnefopam: 10–15 hours[1] |
| Excretion | Urine: 79.3%[1] Feces: 13.4%[1] |
| Identifiers | |
| |
| CAS Number | |
| PubChem CID | |
| ChemSpider | |
| UNII | |
| KEGG | |
| ChEBI | |
| CompTox Dashboard (EPA) | |
| ECHA InfoCard | 100.033.757 |
| Chemical and physical data | |
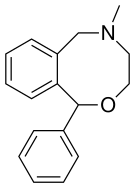
| Formula | C17H19NO |
| Molar mass | 253.345 g·mol−1 |
| 3D model (JSmol) | |
| |
| |
| | |
Nefopam, sold under the brand name Acupan among others, is a centrally acting, non-opioid painkilling medication, that is primarily used to treat moderate to severe pain.[3]
Nefopam acts in the brain and spinal cord to relieve pain via novel mechanisms: antinociceptive effects from triple monoamine reuptake inhibition, and antihyperalgesic activity through modulation of glutamatergic transmission.[4]
Medical uses[edit]
Nefopam is effective for prevention of shivering during surgery or recovery from surgery.[5][6] Nefopam was significantly more effective than aspirin as an analgesic in one clinical trial,[7] although with a greater incidence of side effects such as sweating, dizziness and nausea, especially at higher doses.[8][9] The estimated relative potency of nefopam to morphine indicates that 20 mg of nefopam HCl is the approximate analgesic equal of 12 mg of morphine with comparable analgesic efficacy to morphine,[10][11][12] or oxycodone,[13] while nefopam tends to produce fewer side effects, does not produce respiratory depression,[14] and has much less abuse potential, and so is useful either as an alternative to opioid analgesics, or as an adjunctive treatment for use alongside opioids or other types of analgesics.[12][15] Nefopam is also used to treat severe hiccups.[16] Use of nefopam is also used for the manufacture of a medicament for the treatment of an affective disorder and attention-deficit disorder.[17] Nefopam can also be used as a contradiction for the treatment of Parkinson’s disease. https://www.researchgate.net/publication/228540898_Biological_Peculiarities_of_the_Analgesic_Drug_Nefopam_in_Rats
Contraindications[edit]
Nefopam is contraindicated in people with convulsive disorders, those that have received treatment with irreversible monoamine oxidase inhibitors such as phenelzine, tranylcypromine or isocarboxazid within the past 30 days and those with myocardial infarction pain, mostly due to a lack of safety data in these conditions.[18]
Side effects[edit]
Common side effects include nausea, nervousness, dry mouth, light-headedness and urinary retention.[18] Less common side effects include vomiting, blurred vision, drowsiness, sweating, insomnia, headache, confusion, hallucinations, tachycardia, aggravation of angina and rarely a temporary and benign pink discolouration of the skin or erythema multiforme.[18]
Overdose[edit]
Overdose and death have been reported with nefopam.[19] Overdose usually manifests with convulsions, hallucinations, tachycardia, and hyperdynamic circulation.[18] Treatment is usually supportive, managing cardiovascular complications with beta blockers and limiting absorption with activated charcoal.[18]
Interactions[edit]
It has additive anticholinergic and sympathomimetic effects with other agents with these properties.[18] Its use should be avoided in people receiving some types of antidepressants (tricyclic antidepressants or monoamine oxidase inhibitors) as there is the potential for serotonin syndrome or hypertensive crises to result.[18]
Pharmacology[edit]
| Site | Ki (nM) |
|---|---|
| SERT | 29 |
| NET | 33 |
| DAT | 531 |
| 5-HT2A | 1,685 |
| 5-HT2B | 330 |
| 5-HT2C | 56 |
Pharmacodynamics[edit]
The mechanism of action of nefopam and its analgesic effects are not well understood, although inhibition of the reuptake of serotonin, norepinephrine, and to a lesser extent dopamine (that is, acting as an SNDRI) is thought to be involved.[22][4] It also reduces glutamate signaling via modulating sodium and calcium channels.[23][4]
Pharmacokinetics[edit]
The absolute bioavailability of nefopam is low.[1] It is reported to achieve therapeutic plasma concentrations between 49 and 183 nM.[21] The drug is approximately 73% protein-bound across a plasma range of 7 to 226 ng/mL (28–892 nM).[1] The metabolism of nefopam is hepatic, by N-demethylation and via other routes.[1] Its terminal half-life is 3 to 8 hours, while that of its active metabolite, desmethylnefopam, is 10 to 15 hours.[1] It is eliminated mostly in urine, and to a lesser extent in feces.[1]
Chemistry[edit]
Nefopam is a cyclized analogue of orphenadrine, diphenhydramine, and tofenacin, with each of these compounds different from one another only by the presence of one or two carbons.[24][25][26] The ring system of nefopam is a benzoxazocine system.[24][27]
Society and culture[edit]
Recreational use[edit]
Recreational use of nefopam has rarely been reported,[19] and is far less common than with opioid analgesics.[28]
Names[edit]
In the 1960s, when it was first developed, it had the generic name fenazoxine.[23]
See also[edit]
References[edit]
- ^ a b c d e f g h i j k l m Sanga M, Banach J, Ledvina A, Modi NB, Mittur A (November 2016). "Pharmacokinetics, metabolism, and excretion of nefopam, a dual reuptake inhibitor in healthy male volunteers". Xenobiotica; the Fate of Foreign Compounds in Biological Systems. 46 (11): 1001–16. doi:10.3109/00498254.2015.1136989. PMID 26796604. S2CID 34603935.
- ^ Seyffart G (6 December 2012). Drug Dosage in Renal Insufficiency. Springer Science & Business Media. pp. 407–. ISBN 978-94-011-3804-8.
- ^ Brayfield A, ed. (27 October 2016). "Nefopam hydrochloride". MedicinesComplete. London, UK: Pharmaceutical Press. Retrieved 4 September 2017.
- ^ a b c Girard P, Chauvin M, Verleye M (January 2016). "Nefopam analgesia and its role in multimodal analgesia: A review of preclinical and clinical studies". Clinical and Experimental Pharmacology & Physiology. 43 (1): 3–12. doi:10.1111/1440-1681.12506. PMID 26475417.
- ^ Kang P, Park SK, Yoo S, Hur M, Kim WH, Kim JT, Bahk JH (January 2019). "Comparative effectiveness of pharmacologic interventions to prevent shivering after surgery: a network meta-analysis". Minerva Anestesiologica. 85 (1): 60–70. doi:10.23736/S0375-9393.18.12813-6. PMID 30226340. S2CID 52288008.
- ^ Alfonsi P, Adam F, Passard A, Guignard B, Sessler DI, Chauvin M (January 2004). "Nefopam, a nonsedative benzoxazocine analgesic, selectively reduces the shivering threshold in unanesthetized subjects". Anesthesiology. 100 (1): 37–43. doi:10.1097/00000542-200401000-00010. PMC 1283107. PMID 14695722.
- ^ Cohen A, Hernandez CM (1976). "Nefopam hydrochloride: new analgesic agent". The Journal of International Medical Research. 4 (2): 138–43. doi:10.1177/030006057600400211. PMID 799984. S2CID 41618926.
- ^ Wang RI, Waite EM (July 1979). "The clinical analgesic efficacy of oral nefopam hydrochloride". Journal of Clinical Pharmacology. 19 (7): 395–402. doi:10.1002/j.1552-4604.1979.tb02498.x. PMID 479385. S2CID 25877487.
- ^ Pillans PI, Woods DJ (September 1995). "Adverse reactions associated with nefopam". The New Zealand Medical Journal. 108 (1008): 382–4. PMID 7566787.
- ^ Sunshine A, Laska E (November 1975). "Nefopam and morphine in man". Clinical Pharmacology and Therapeutics. 18 (5 Pt 1): 530–4. doi:10.1002/cpt1975185part1530. PMID 1102231. S2CID 19051105.
- ^ Phillips G, Vickers MD (October 1979). "Nefopam in postoperative pain". British Journal of Anaesthesia. 51 (10): 961–5. doi:10.1093/bja/51.10.961. PMID 391253.
- ^ a b Heel RC, Brogden RN, Pakes GE, Speight TM, Avery GS (April 1980). "Nefopam: a review of its pharmacological properties and therapeutic efficacy". Drugs. 19 (4): 249–67. doi:10.2165/00003495-198019040-00001. PMID 6991238. S2CID 24713213.
- ^ Tigerstedt I, Tammisto T, Leander P (December 1979). "Comparison of the analgesic dose-effect relationships of nefopam and oxycodone in postoperative pain". Acta Anaesthesiologica Scandinavica. 23 (6): 555–60. doi:10.1111/j.1399-6576.1979.tb01486.x. PMID 397711. S2CID 40976610.
- ^ Gasser JC, Bellville JW (August 1975). "Respiratory effects of nefopam". Clinical Pharmacology and Therapeutics. 18 (2): 175–9. doi:10.1002/cpt1975182175. PMID 1097153. S2CID 22220503.
- ^ Kapfer B, Alfonsi P, Guignard B, Sessler DI, Chauvin M (January 2005). "Nefopam and ketamine comparably enhance postoperative analgesia". Anesthesia and Analgesia. 100 (1): 169–74. doi:10.1213/01.ANE.0000138037.19757.ED. PMC 1283103. PMID 15616073.
- ^ Bilotta F, Rosa G (December 2000). "Nefopam for severe hiccups". The New England Journal of Medicine. 343 (26): 1973–4. doi:10.1056/nejm200012283432619. PMID 11186682.
- ^ WO2007012870A2, Lyne, Michael Harvey & Bannister, Robin Mark, "Use of nefopam for the treatment of affective disorders", issued 2007-02-01
- ^ a b c d e f g "Data Sheet ACUPAN™ Nefopam hydrochloride 30 mg tablets 20 mg intramuscular injection" (PDF). Medsafe New Zealand. iNova Pharmaceuticals (New Zealand) Limited. 3 September 2007. Retrieved 10 March 2014.
- ^ a b Bismuth C, Fournier PE, Bavoux E, Husson O, Lafon D (September 1987). "[Chronic abuse of the analgesic nefopam (Acupan)]". Journal de Toxicologie Clinique et Expérimentale (in French). 7 (5): 343–6. PMID 3448182.
- ^ Roth BL, Driscol J. "PDSP Ki Database". Psychoactive Drug Screening Program (PDSP). University of North Carolina at Chapel Hill and the United States National Institute of Mental Health. Retrieved 14 August 2017.
- ^ a b Gregori-Puigjané E, Setola V, Hert J, Crews BA, Irwin JJ, Lounkine E, et al. (July 2012). "Identifying mechanism-of-action targets for drugs and probes" (PDF). Proceedings of the National Academy of Sciences of the United States of America. 109 (28): 11178–83. Bibcode:2012PNAS..10911178G. doi:10.1073/pnas.1204524109. PMC 3396511. PMID 22711801.
- ^ "New Zealand Data Sheet Acupan(TM)" (PDF). Medsafe. New Zealand The Ministry of Health. 17 May 2017. Retrieved 4 September 2017.
- ^ a b Kim KH, Abdi S (April 2014). "Rediscovery of nefopam for the treatment of neuropathic pain". The Korean Journal of Pain. 27 (2): 103–11. doi:10.3344/kjp.2014.27.2.103. PMC 3990817. PMID 24748937.
- ^ a b Wermuth CG, Aldous D, Raboisson P, Rognan D (1 July 2015). The Practice of Medicinal Chemistry. Elsevier Science. pp. 250–251. ISBN 978-0-12-417213-5.
- ^ Sneader W (23 June 2005). Drug Discovery: A History. John Wiley & Sons. pp. 405–. ISBN 978-0-471-89979-2.
- ^ Kubinyi H, Müller G (6 March 2006). Chemogenomics in Drug Discovery: A Medicinal Chemistry Perspective. John Wiley & Sons. pp. 54–. ISBN 978-3-527-60402-9.
- ^ Cruz A (2014). Therapeutic Hypothermia. CRC Press. pp. 176–.
- ^ Tracqui A, Berthelon L, Ludes B (May 2002). "Fatal overdosage with nefopam (Acupan)". Journal of Analytical Toxicology. 26 (4): 239–43. doi:10.1093/jat/26.4.239. PMID 12054367.