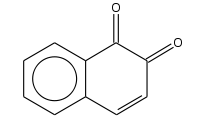
| |
| Names | |
|---|---|
| Preferred IUPAC name
Naphthalene-1,2-dione | |
| Other names
o-Naphthoquinone,
β-naphthoquinone | |
| Identifiers | |
3D model (JSmol)
|
|
| ChEBI | |
| ChEMBL | |
| ChemSpider | |
| ECHA InfoCard | 100.007.602 |
| KEGG | |
PubChem CID
|
|
| UNII | |
CompTox Dashboard (EPA)
|
|
| |
| |
| Properties | |
| C10H6O2 | |
| Molar mass | 158.156 g·mol−1 |
| Appearance | yellow solid |
| Melting point | 145 to 147 °C (293 to 297 °F; 418 to 420 K) |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa).
| |
1,2-Naphthoquinone or ortho-naphthoquinone is a polycyclic aromatic organic compound with formula C
10H
6O
2. This yellow solid is prepared by oxidation of 1-amino-2-hydroxynaphthalene with ferric chloride.[1]
Occurrence[edit]
This diketone (an ortho-quinone) is a metabolite of naphthalene. It arises from the naphthalene-1,2-oxide.[2]
It is also found in diesel exhaust particles. The accumulation of this toxic metabolite in rats from doses of naphthalene has been shown to cause eye damage, including the formation of cataracts.[3]
See also[edit]
- 1,4-Naphthoquinone, an isomer of 1,2-naphthoquinone
References[edit]
- ^ Louis F. Fieser (1937). "1,2-Naphthoquinone". Org. Synth. 17: 68. doi:10.15227/orgsyn.017.0068.
- ^ Yoshito Kumagai; Yasuhiro Shinkai; Takashi Miura; Arthur K. Cho (2011). "The Chemical Biology of Naphthoquinones and Its Environmental Implications". Annual Review of Pharmacology and Toxicology. 52: 221–47. doi:10.1146/annurev-pharmtox-010611-134517. PMID 21942631.
- ^ Qian, W.; Shichi, H. (2001). "Naphthoquinone-Induced Cataract in Mice: Possible Involvement of Ca2+ Release and Calpain Activation". Journal of Ocular Pharmacology and Therapeutics. 17 (4): 383–392. doi:10.1089/108076801753162799. PMID 11572469.
External links[edit]
- Troester, M. A.; Lindstrom, A. B.; Waidyanatha, S.; Kupper, L. L.; Rappaport, S. M. (2002). "Stability of Hemoglobin and Albumin Adducts of Naphthalene Oxide, 1,2-Naphthoquinone, and 1,4-Naphthoquinone". Toxicological Sciences. 68 (2): 314–321. doi:10.1093/toxsci/68.2.314. PMID 12151627.
- Kikuno, S.; Taguchi, K.; Iwamoto, N.; et al. (2006). "1,2-Naphthoquinone Activates Vanilloid Receptor 1 through Increased Protein Tyrosine Phosphorylation, Leading to Contraction of Guinea Pig Trachea". Toxicology and Applied Pharmacology. 210 (1–2): 47–54. doi:10.1016/j.taap.2005.06.015. PMID 16039679.