 | |
| Clinical data | |
|---|---|
| Other names | MOHN; MHN; 4-Hydroxy-17α-methyl-19-nortestosterone; HMNT; 4,17β-Dihydroxy-17α-methylestr-4-en-3-one |
| Routes of administration | By mouth[1] |
| Drug class | Androgen; Anabolic steroid |
| Identifiers | |
| |
| CAS Number | |
| PubChem CID | |
| ChemSpider | |
| UNII | |
| Chemical and physical data | |
| Formula | C19H28O3 |
| Molar mass | 304.430 g·mol−1 |
| 3D model (JSmol) | |
| |
| |
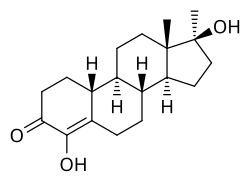
Methylhydroxynandrolone (MOHN, MHN), also known as 4-hydroxy-17α-methyl-19-nortestosterone (HMNT), as well as 4,17β-dihydroxy-17α-methylestr-4-en-3-one, is a synthetic, orally active anabolic–androgenic steroid (AAS) and a 17α-alkylated derivative of nandrolone (19-nortestosterone) which was never marketed.[1] It was first described in 1964 and was studied in the treatment of breast cancer, but was not introduced for clinical use.[1][2] The drug re-emerged in 2004 when it started being sold on the Internet as a "dietary supplement".[1] MOHN joined other AAS as a controlled substance in the United States on 20 January 2005.[1]
MOHN is non-aromatizable due to the presence of a hydroxy group at the C4 position, and for this reason, poses no risk of estrogenic side effects like gynecomastia at any dosage, unlike many other AAS.[1] 5α-Reduction is also inhibited by the C4 hydroxy group of MOHN and, because of this, MOHN may have a relatively higher ratio of androgenic to anabolic activity than other nandrolone derivatives (as 5α-reduction, opposite to the case of most other AAS, decreases AAS potency for most nandrolone derivatives).[1] Early assays found that MOHN had approximately 13 times the anabolic activity and 3 times the androgenic activity of methyltestosterone.[1]
MOHN is the 4-hydroxylated derivative of normethandrone (17α-methyl-19-nortestosterone), the 17α-methylated derivative of oxabolone (4-hydroxy-19-nortestosterone), the 4-hydroxylated and 17α-methylated derivative of nandrolone (19-nortestosterone), and the 19-demethylated analogue of oxymesterone (4-hydroxy-17α-methyltestosterone).[1]
See also[edit]
References[edit]
- ^ a b c d e f g h i William Llewellyn (1 November 2008). Anabolics: Anabolic Steroid Reference Guide. William Llewellyn. p. 311. ISBN 978-0-9679304-7-3.
- ^ Di Pietro S, Salvadori B (1964). "Sperimentazione Clinica del 4-Idrossi-17-alfa-metil-19-nortestosterone nel Carcinoma Mammario Diffuso" [Clinical Trial with 4-Hydroxy-17α-methyl-19-nortestosterone in Advanced Breast Cancer]. Tumori (in Italian). 50 (6): 445–456. doi:10.1177/030089166405000602. ISSN 0300-8916. PMID 14261594. S2CID 208183701.
External links[edit]