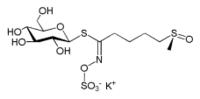
 Potassium salt of glucoraphanin
| |
| Names | |
|---|---|
| IUPAC name
1-S-[(1E)-5-(methylsulfinyl)-N-(sulfonatooxy)pentanimidoyl]-1-thio-β-D-glucopyranose
| |
| Other names
Glucorafanin; 4-Methylsulfinylbutyl glucosinolate, Sulforaphane glucosinolate
| |
| Identifiers | |
3D model (JSmol)
|
|
| ChEBI | |
| ChemSpider | |
PubChem CID
|
|
| UNII | |
CompTox Dashboard (EPA)
|
|
| |
| |
| Properties | |
| C12H23NO10S3 | |
| Molar mass | 437.49 g·mol−1 |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa).
| |
Glucoraphanin is a glucosinolate found in broccoli,[1][2] mustard and other cruciferous vegetables.[3][4]
Glucoraphanin is converted to sulforaphane by the enzyme myrosinase.[5] In plants, sulforaphane deters insect predators and acts as a selective antibiotic.[6]
Synthesis[edit]
Glucoraphanin is derived from dihomomethionine, which is methionine chain-elongated twice.[7] The sulfinyl group is chiral, and has R absolute configuration. The stereochemistry is set when an oxygen atom added to 4-methylthiobutylglucosinolate by a flavin monooxygenase.[8]
Research[edit]
Sulforaphane and other isothiocyanates have been studied for their potential biological effects.[3] The isothiocyanates formed from glucosinolates are under laboratory research to assess the expression and activation of enzymes that metabolize xenobiotics, such as carcinogens.[3] Observational studies have been conducted to determine if consumption of cruciferous vegetables affects cancer risk in humans, but there is insufficient clinical evidence to indicate that consuming glucoraphanin and other isothiocyanates in cruciferous vegetables is beneficial, according to a 2017 review.[3]
Plant breeding[edit]
Cultivars of broccoli have been bred to contain two to three times more glucoraphanin than standard broccoli.[9] Romanesco broccoli may contain up to ten times more glucoraphanin than typical broccoli varieties.[10] Frostara, Black Tuscany, and red cabbage also contain higher levels of glucoraphanin than broccoli.[10]
References[edit]
- ^ James, D.; Devaraj, S.; Bellur, P.; Lakkanna, S.; Vicini, J.; Boddupalli, S. (2012). "Novel concepts of broccoli sulforaphanes and disease: Induction of phase II antioxidant and detoxification enzymes by enhanced-glucoraphanin broccoli". Nutrition Reviews. 70 (11): 654–65. doi:10.1111/j.1753-4887.2012.00532.x. PMID 23110644.
- ^ Jeffery, E. H.; Brown, A. F.; Kurilich, A. C.; Keck, A. S.; Matusheski, N.; Klein, B. P.; Juvik, J. A. (2003). "Variation in content of bioactive components in broccoli". Journal of Food Composition and Analysis. 16 (3): 323–330. doi:10.1016/S0889-1575(03)00045-0.
- ^ a b c d "Isothiocyanates". Micronutrient Information Center, Linus Pauling Institute, Oregon State University. 1 April 2017. Retrieved 26 June 2022.
- ^ Oh, K.; SangOk, K.; Rak, C. (2015). "Sinigrin content of different parts of Dolsan leaf mustard". Korean Journal of Food Preservation. 22 (4): 553–558. doi:10.11002/kjfp.2015.22.4.553.
- ^ Cuomo, Valentina; Luciano, Fernando B.; Meca, Giuseppe; Ritieni, Alberto; Mañes, Jordi (26 November 2014). "Bioaccessibility of glucoraphanin from broccoli using an gastrointestinal digestion model". CyTA - Journal of Food. 13 (3): 361–365. doi:10.1080/19476337.2014.984337. S2CID 96578211.
- ^ Fahey, Jed W.; Holtzclaw, W. David; Wehage, Scott L.; Wade, Kristina L.; Stephenson, Katherine K.; Talalay, Paul; Mukhopadhyay, Partha (2 November 2015). "Sulforaphane Bioavailability from Glucoraphanin-Rich Broccoli: Control by Active Endogenous Myrosinase". PLOS ONE. 10 (11): e0140963. Bibcode:2015PLoSO..1040963F. doi:10.1371/journal.pone.0140963. PMC 4629881. PMID 26524341.
- ^ Agerbirk N, Olsen CE (May 2012). "Glucosinolate structures in evolution". Phytochemistry. 77: 16–45. Bibcode:2012PChem..77...16A. doi:10.1016/j.phytochem.2012.02.005. PMID 22405332.
- ^ Fredd Vergara; et al. (Nov 2008). "Determination of the absolute configuration of the glucosinolate methyl sulfoxide group reveals a stereospecific biosynthesis of the side chain". Phytochemistry. 69 (15): 2737–2742. Bibcode:2008PChem..69.2737V. doi:10.1016/j.phytochem.2008.09.008. PMID 18945459.
- ^ Cheng, Maria (October 26, 2011). "UK scientists grow super broccoli". Boston.com. Associated Press. Archived from the original on 3 June 2012. Retrieved 10 November 2011.
{{cite news}}: CS1 maint: bot: original URL status unknown (link) - ^ a b Hahn, Christoph; Müller, Anja; Kuhnert, Nikolai; Albach, Dirk (2016-04-27). "Diversity of Kale (Brassica oleracea var. sabellica): Glucosinolate Content and Phylogenetic Relationships". Journal of Agricultural and Food Chemistry. 64 (16): 3215–3225. doi:10.1021/acs.jafc.6b01000. ISSN 0021-8561. PMID 27028789.