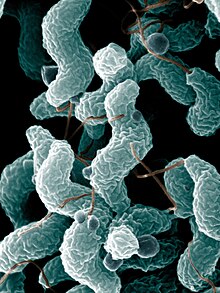
| Campylobacter jejuni | |
|---|---|
| |
| Scientific classification | |
| Domain: | Bacteria |
| Phylum: | Campylobacterota |
| Class: | "Campylobacteria" |
| Order: | Campylobacterales |
| Family: | Campylobacteraceae |
| Genus: | Campylobacter |
| Species: | C. jejuni
|
| Binomial name | |
| Campylobacter jejuni (Jones et al., 1931) Veron & Chatelain, 1973
| |
Campylobacter jejuni is a species of pathogenic bacteria that is commonly associated with poultry, and is also often found in animal feces. This species of microbe is one of the most common causes of food poisoning in Europe and in the US, with the vast majority of cases occurring as isolated events rather than mass outbreaks. Active surveillance through the Foodborne Diseases Active Surveillance Network (FoodNet) indicates that about 20 cases are diagnosed each year for each 100,000 people in the US, while many more cases are undiagnosed or unreported; the CDC estimates a total of 1.5 million infections every year.[1] The European Food Safety Authority reported 246,571 cases in 2018, and estimated approximately nine million cases of human campylobacteriosis per year in the European Union.[2] Campylobacter jejuni infections are increasing at an alarming rate in Europe, North America, and Australia. In Africa, Asia, and the Middle East, data indicates that C. jejuni infections are endemic.[3]
Campylobacter is a genus of bacteria that is among the most common causes of bacterial infections in humans worldwide. Campylobacter means "curved rod", deriving from the Greek kampylos (curved) and baktron (rod). Of its many species, C. jejuni is considered one of the most important from both a microbiological and public health perspective.[4][5]
C. jejuni is commonly associated with poultry, and is also commonly found in animal feces. Campylobacter is a helical-shaped, non-spore-forming, Gram-negative, microaerophilic, nonfermenting motile bacterium with a single flagellum at one or both poles,[6] which are also oxidase-positive and grow optimally at 37 to 42 °C.[7][8][9][10] When exposed to atmospheric oxygen, C. jejuni is able to change into a coccal form.[11] This species of pathogenic bacteria is one of the most common causes of human gastroenteritis in the world. Food poisoning caused by Campylobacter species can be severely debilitating, but is rarely life-threatening. It has been linked with subsequent development of Guillain–Barré syndrome, which usually develops two to three weeks after the initial illness.[12] Individuals with recent C. jejuni infections develop Guillain-Barré syndrome at a rate of 0.3 per 1000 infections, about 100 times more often than the general population.[13] Another chronic condition that may be associated with campylobacter infection is reactive arthritis.[14] Reactive arthritis is a complication strongly associated with a particular genetic make-up. That is, persons who have the human leukocyte antigen B27 (HLA-B27) are most susceptible. Most often, the symptoms of reactive arthritis will occur up to several weeks after infection.[4][15]
History[edit]
Campylobacter jejuni was originally named Vibrio jejuni due to its likeness to Vibrio spp. until 1963. Seabald and Vernon proposed the genus Campylobacter due to its low levels of guanine and cytosine, non-fermentative metabolism, and microaerophilic growth requirements.[16] In 1886 a pediatrician, Theodor Escherich, observed campylobacter from diarrhea samples of children.[17] The first isolation of C. jejuni was in Brussels, Belgium, from stool samples of a patient with diarrhea.[17] The first well recorded incident of campylobacter infection occurred in 1938. Campylobacter found in milk caused diarrhea among 355 inmates in two state institutions in Illinois.[16]
Metabolism[edit]
C. jejuni is unable to utilize sugars as a carbon source, primarily using amino acids for growth instead.[18] The main reason C. jejuni lacks glycolytic capabilities is a lack of glucokinase[19] and a lack of the 6-phosphofructokinase enzyme to employ the EMP pathway.[16] The four main amino acids C. jejuni takes in are serine, aspartate, asparagine, and glutamate, which are listed in order of preference.[16] If all of these are depleted, some strains can use proline as well.[16] Either the host or metabolic activity of other gut microbes can supply these amino acids.[18]
The metabolic pathways C. jejuni is capable of include the TCA cycle, a non-oxidative pentose phosphate pathway, gluconeogenesis, and fatty acid synthesis.[20] Serine is the most important amino acid used for growth, brought into the cell by SdaC transport proteins and further broken down into pyruvate by the SdaA dehydratase.[19] Though this pyruvate cannot directly be converted into phosphoenolpyruvic acid (as C. jejuni lacks this synthetase), the pyruvate can enter the TCA cycle to form oxaloacetic acid intermediates that can be converted to phosphoenolpyruvic acid for gluconeogenesis.[20] This production of carbohydrates is important for the virulence factors of C. jejuni.[20] The pyruvate created from serine can also be converted to acetyl CoA and be applied to fatty acid synthesis or continue into the TCA cycle to create precursors for other biosynthetic pathways.[20] Aspartate and glutamate are both brought into the cell via Peb1A transport proteins.[19] Glutamate can be transaminated into aspartate, and aspartate can be deaminated to make fumerate that feeds into the TCA cycle as well.[19] Asparagine is also able to be deaminated into aspartate (which follows the process into the TCA cycle mentioned above).[19] While the amino acids listed above are able to be metabolized, C. jejuni is capable of taking in many of the other amino acids which helps to lower the anabolic cost of de novo synthesis.[20]
If other sources of carbon are exhausted, C. jejuni can also utilize acetate and lactate as carbon sources.[19] Acetate is a normal secreted byproduct of C. jejuni metabolism stemming from the recycling of CoA, and the absence of other carbon sources can cause C. jejuni to "switch" this reaction to take in acetate for the conversion to acetyl-CoA (catalyzed by phosphate acetyltransferase and acetate kinase enzymes).[19][20] Lactate is a normal byproduct of many fermentative bacteria in the gut, and C. jejuni can take in and oxidize this lactate to supply pyruvate through the activity of dehydrogenase iron-sulfur enzyme complexes.[19]
The energetic needs of these anabolic pathways are met in multiple ways. The cytochrome c and quinol terminal oxidases allow for C. jejuni to use oxygen as a terminal electron acceptor for the reduced carriers produced through the TCA cycle (hence why C. jejuni is considered an obligate microaerophile).[21] The conversion of acetyl-CoA to acetate mentioned above has substrate-level phosphorylation take place, giving another form of energy production without the use of microaerophilic respiration.[20]
C. jejuni can utilize many different electron donors for its metabolic processes, using NADH and FADH most commonly - though C. jejuni utilizes NADH poorly compared to FADH due to a replacement of genes encoding subunits for NADH dehydrogenases for genes contributing to processes relating to FADH electron donation.[22] Aside from these donors, C. jejuni can turn to products from the host gut microbiota including hydrogen, lactate, succinate, and formate to contribute electrons; formate, for example, is generated through intestinal mixed-acid fermentation.[22] Unlike almost all other Campylobacter or Helicobacter species, C. jejuni can also accept electrons from sulfite and metabisulfite through its cytochrome c oxidoreductase system.[22]
While oxygen is mainly used as a terminal electron acceptor, C. jejuni can use nitrate, nitrite, sulfur oxides (such as dimethyl sulfoxide or trimethylamine N-oxide), or fumarate as terminal electron acceptors as well to survive as a microaerophilic bacterium.[22] Due to oxygen-limited conditions in the common areas of colonization, C. jejuni possesses two separate terminal oxidases with different affinities for oxygen, where the low affinity oxidase can directly retrieve electrons from menaquinones.[22] The adaptations allowing for multiple electron acceptors help to combat the problem with reactive oxygen species arising from the sole use of oxygen as well; C. jejuni cannot grow under strictly aerobic conditions. Enzymes C. jejuni carries to impede the effects of reactive oxygen species include: superoxide dismutase SodB, alkyl hydroxide reductase AhpC, catalase KatA, and thiol peroxidases Tpx and Bcp.[22]
Disease[edit]
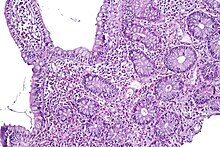
Campylobacteriosis is an infectious disease caused by bacteria of the genus Campylobacter. In most patients presenting with campylobacteriosis, symptoms develop within two to five days of exposure to the organism and illness typically lasts seven days following onset.[23] Infection with C. jejuni typically results in enteritis, or inflammation of the small intestine, which is characterized by abdominal pain, voluminous diarrhea (often bloody), fever, and malaise. Individuals infected with this bacteria can experience a prodromal phase of symptoms for the first 1 to 3 days, in which the more severe portion of the disease occurs. The prodromal phase presents with symptoms including rigors, high fever, body aches, and dizziness. Other than the prodromal phase, the acute diarrheal phase of enteritis usually lasts around 7 days, however abdominal pain can persist for weeks afterward.[3] The disease is usually self-limiting; however, it does respond to antibiotics. Severe (accompanying fevers, blood in stools) or prolonged cases may require erythromycin, azithromycin, ciprofloxacin, or norfloxacin. Fluid replacement via oral rehydration salts may be needed and intravenous fluid may be required for serious cases.[23] Possible complications of campylobacteriosis include Guillain–Barré syndrome and reactive arthritis.[3]
Pathogenesis[edit]
C. jejuni employs unique strategies to breach the intestinal epithelial layer of its host cells. [24] It uses proteases, particularly HtrA, to cleverly disrupt cell junctions and temporarily traverse the cells.[24] The membrane-bound protein Fibronectin is a critical binding site for C. jejuni on the basolateral side of the polarized epithelial cell, facilitating this process.[24] Once inside the cell, C. jejuni leverages dynein to access the perinuclear space within the Clathrin-Coated Vesicle, avoiding lysosomal digestion for up to 72 hours.[24]
To initiate infection, C. jejuni must penetrate the gut enterocytes.[25] C. jejuni releases several different toxins, mainly enterotoxin and cytotoxins, which vary from strain to strain and correlate with the severity of the enteritis (inflammation of the small intestine). During infection, levels of all immunoglobulin classes rise. Of these, IgA is the most important because it can cross the gut wall. IgA immobilises organisms, causing them to aggregate and activate complement, and also gives short-term immunity against the infecting strain of organism.[26] The bacteria colonize the small and large intestines, causing inflammatory diarrhea with fever. Stools contain leukocytes and blood. The role of toxins in pathogenesis is unclear. C jejuni antigens that cross-react with one or more neural structures may be responsible for triggering the Guillain–Barré syndrome.[10]
Hypoacylated lipopolysaccharide (LPS) from C. jejuni induces moderate TLR4-mediated inflammatory response in macrophages and such LPS bioactivity may eventually result in the failure of local and systemic bacterial clearance in patients. At the same time, moderation of anti-bacterial responses may be advantageous for infected patients in clinical practice, since such an attenuated LPS may not be able to induce severe sepsis in susceptible individuals.[27]
One of the most important virulence factors of C. jejuni are flagella. The flagellar protein FlaA has been proven to be one of the abundant proteins in the cell. Flagella are required for motility, biofilm formation, host cell interactions and host colonization. The flagella in C. jejuni can also aid in the secretion intracellular proteins.[18] The production of flagella is energetically costly so the production must be regulated from metabolic standpoint. CsrA is a post-transcriptional regulator that regulates the expression of FlaA by binding to flaA mRNA and is able to repress its translation. CsrA mutant strains have been studied and the mutant strains exhibit dysregulation of 120-150 proteins that are included in motility, host cell adherence, host cell invasion, chemotaxis, oxidative stress resistance, respiration and amino acid and acetate metabolism. Transcriptional and post-transcriptional regulation of flagellar synthesis in C. jejuni enables proper biosynthesis of flagella and it is important for pathogenesis of this bacteria.[28]
C. jejuni employs a highly sophisticated navigation system called chemotaxis.[24] This system is crucial when the bacterium requires guidance through chemical signals.[24] The chemotaxis system utilizes specific chemoattractants that direct the bacterium toward areas with a higher concentration of the attractants.[24] The exact nature of chemoattractants is dependent on the surrounding environmental conditions.[24] Additionally, when the bacterium needs to move away, it uses negative chemotaxis to move in the opposite direction.[24]
Other important virulence factors of C. jejuni are the ability to produce N-linked glycosylation of more than 30 proteins. These proteins are important for the bacteria colonization, adherence and invasion. C. jejuni secretes campylobacter invasive antigens (Cia) which facilitates the motility. The bacteria produces also cytolethal distending toxins that participate in cell cycle control and induction of host cell apoptosis. C. jejuni also exploits different adaptation strategies in which the host factors seem to play a role for pathogenesis of this bacteria.[29]
DNA repair[edit]
In the intestines, bile functions as a defensive barrier against colonization by C. jejuni.[30][31] When C. jejuni is grown in a medium containing the bile acid deoxycholic acid, a component of bile, the DNA of C. jejuni is damaged by a process involving oxidative stress.[32][30] To survive, C. jejuni cells repair this DNA damage by a system employing proteins AddA and AddB that are needed for repair of DNA double-strand breaks.[30]
C.jejuni uses homologous recombination to repair its DNA, facilitated by the AddA and AddB proteins.[30] These proteins replace RecBCD, which is used in other bacteria like E.Coli.[30] AddA and AddB are crucial for nuclease, helicase, and Chi recognition,[33] which allow for successful homologous recombination.[30]
When AddA and AddB are introduced into a wild C.jejuni variant, an added deletion mutant gene addAB gene is formed, which repairs DNA damaged by oxidative stress.[30] This inclusion protects C.jejuni from deoxycholate found in bile, allowing for survival.[30] However, the added gene is absent during growth in deoxycholate from 10 to 16 hours and may be up-regulated in response to environmental conditions.[30] Additionally, AddAB proteins enhance C.jejuni colonization of chicken intestines.[30]
Immune response[edit]
Campylobacter jejuni infection and eventual destruction of host cell releases chemokines that cause inflammation and activate immune response cells. Inflammatory chemokines such as CXCL1, CCL3/CCL4, CCL2, and CXCL10 are upregulated, further triggering the immune response. The immune response activation is primarily driven by the use of ADP-heptoses to activate ALPK1,[34] by a C. jejuni infection[24]
Neutrophil granulocytes use phagocytosis to combat C. jejuni infection, releasing antimicrobial proteins and proinflammatory substances. However, C. jejuni can influence the differentiation process of specific types of neutrophil granulocytes, triggering hypersegmentation and increased reactivity, which leads to delayed apoptosis and higher production of reactive oxygen species. In experimental processes, T cells from an immune response only start to grow in number at the inflammation site from the seventh day after infection.[24]
After 11 days of having a Campylobacter jejuni infection, the B lymphocytes in the body increase the production of antibodies that specifically fight against C. jejuni flagellin.[24] The persistence of these antibodies in the body can last up to one-year post-infection. In this case, the development of Guillain-Barré syndrome (GBS) is associated with autoimmune IgG1 antibodies.[24]
Campylobacter infections often precede GBS, indicating that molecular mimicry between the bacteria and host nervous tissues may be the underlying cause.[24] C. jejuni , the most common causative agent of human campylobacteriosis, can survive in the gut for several days but does not establish a long-term infection due to its low replication rate, which is incompatible with a persistent bacterial presence.[24] The bacteria-induced apoptosis of infected gut cells results in the rapid clearance of the pathogen, which likely contributes to the self-limiting nature of the disease.[24]
Sources[edit]
Campylobacter jejuni is commonly associated with poultry, and it naturally colonises the digestive tract of many bird species. All types of poultry and wild birds can become colonized with campylobacter. One study found that 30% of European starlings in farm settings in Oxfordshire, United Kingdom, were carriers of C. jejuni. It is also common in cattle, and although it is normally a harmless commensal of the gastrointestinal tract in these animals, it can cause campylobacteriosis in calves. It has also been isolated from wombat and kangaroo feces, being a cause of bushwalkers' diarrhea. Contaminated drinking water and unpasteurized milk provide an efficient means for distribution. Contaminated food is a major source of isolated infections, with incorrectly prepared meat and poultry as the primary source of the bacteria.[35] Moreover, surveys show that 20 to 100% of retail chickens are contaminated. This is not overly surprising, since many healthy chickens carry these bacteria in their intestinal tracts and often in high concentrations, up to 108 cfu/g.[36] The bacteria contaminate the carcasses due to poor hygiene during the slaughter process. Several studies have shown increased concentrations of campylobacter on the carcasses after the evisceration.[37][36] Studies have investigated the chicken microbiome to understand how, why and when campylobacter appears within the chicken gut.[38] The impact of industrial system production systems on the chicken gut microbiome and campylobacter prevalence have also been investigated.[39]
Raw milk is also a source of infections. The bacteria are often carried by healthy cattle and by flies on farms. Unchlorinated water may also be a source of infections. However, properly cooking chicken, pasteurizing milk, and chlorinating drinking water kill the bacteria.[40] While salmonella is transmitted vertically in eggs, campylobacter is not. Therefore, consumption of eggs does result in human infection from campylobacter.[41]
Complications[edit]
Local complications of campylobacter infections occur as a result of direct spread from the gastrointestinal tract and can include cholecystitis, pancreatitis, peritonitis, and massive gastrointestinal hemorrhage. Extraintestinal manifestations of campylobacter infection are quite rare and may include meningitis, endocarditis, septic arthritis, osteomyelitis, and neonatal sepsis. Bacteremia is detected in <1% of patients with campylobacter enteritis and is most likely to occur in patients who are immunocompromised or among the very young or very old.[42] Transient bacteremia in immunocompetent hosts with C. jejuni enteritis may be more common but not detected because the killing action rapidly clears most normal human serotypes, and blood cultures are not routinely performed for patients with acute gastrointestinal illness.[43]
Serious systemic illness caused by campylobacter infection rarely occurs, but can lead to sepsis and death. The case-fatality rate for campylobacter infection is 0.05 per 1000 infections. For instance, one major possible complication that C. jejuni can cause is Guillain–Barré syndrome, which induces neuromuscular paralysis in a sizeable percentage of those who suffer from it. Over time, the paralysis is typically reversible to some extent; nonetheless, about 20% of patients with GBS are left disabled, and around 5% die. Another chronic condition that may be associated with campylobacter infection is reactive arthritis.[14] Reactive arthritis is a complication strongly associated with a particular genetic make-up. That is, persons who have the human leukocyte antigen B27 (HLA-B27) are most susceptible. Most often, the symptoms of reactive arthritis will occur up to several weeks after infection.[4][15]
Epidemiology[edit]
Frequency[edit]
United States[edit]
An estimated 2 million cases of campylobacter enteritis occur annually, accounting for 5–7% of cases of gastroenteritis.[44] Campylobacter has a large animal reservoir, with up to 100% of poultry, including chickens, turkeys, and waterfowl, having asymptomatic intestinal infections. The major reservoirs of C. fetus are cattle and sheep. More than 90% of campylobacter infections occur during the summer months due to undercooked meats from outdoor cooking..[3] Nonetheless, the incidence of campylobacter infections has been declining. Changes in the incidence of culture-confirmed Campylobacter infections have been monitored by the Foodborne Diseases Active Surveillance Network (FoodNet) since 1996. In 2010, campylobacter incidence showed a 27% decrease compared with 1996–1998. In 2010, the incidence was 13.6 cases per 100,000 population, and this did not change significantly compared with 2006–2008.[45][46]
Europe[edit]
In 2020, there were around 120,000 cases of C. jejuni infection, which showed a decline of about 25.4% compared to the previous year.[24] However, the COVID-19 pandemic may have influenced this decrease, and its statistical significance is yet to be determined.[24] C. jejuni infections tend to peak in July, which could be linked to the rise in temperature worldwide.[24] This pattern is associated with an increased reflection rate of the bacteria, which needs further investigation to establish any potential correlations.[24]
Globally[edit]
Campylbacter jejuni infections are extremely common worldwide, although exact figures are not available. New Zealand reported the highest national rate, which peaked in May 2006 at 400 per 100,000 population.[1][46] C. jejuni infection is a significant global health issue, with infection rates ranging from 0.3 to 2.9%.[24] It is a widespread infection that affects individuals of all ages but is more prevalent in developing countries.[24] In these areas, diarrhea is the most common clinical presentation, and it has a severe impact on children.[24]
Sex[edit]
Campylobacter is more frequently isolated in males than females, and homosexual men appear to have a higher risk of infection by atypical campylobacter-related species such as Helicobacter cinaedi and Helicobacter fennelliae.[46]
Age[edit]
Campylobacter infections can occur in all age groups. Studies show a peak incidence in children younger than 1 year and in people aged 15–29 years. The age-specific attack rate is highest in young children. In the United States, the highest incidence of Campylobacter infection in 2010 was in children younger than 5 years and was 24.4 cases per 100,000 population.[45] Community based studies done in developing countries show about 60,000 out of every 100,000 children under five years old are affected by campylobacter infections.[3] However, the rate of fecal cultures positive for campylobacter species is greatest in adults and older children.[46]
Diagnosis[edit]
Diagnostic tests are available to identify campylobacter infections, including those caused by C.jejuni.[3] The stool culture is considered the gold standard for diagnosing C.jejuni,[47][better source needed] and selective culture techniques are used to distinguish it from other variants.[3] Stool cultures are grown at 42 degrees Celsius in an atmosphere of 85% N2, 10% CO2, and 5% O2, as C.jejuni requires these conditions due to being thermophilic and microaerophilic.[48] A final diagnosis from a stool sample requires a gram stain or phase contrast microscopy.[47]
Aside from stool cultures, C.jejuni can be detected using enzyme immunoassay (EIA) or polymerase chain reaction (PCR).[3] These methods are more sensitive than stool cultures, but PCR tends to be the most sensitive especially in children and developing countries.[49]
Treatment[edit]
Campylobacter infections tend to be mild, requiring only hydration and electrolyte repletion while diarrhea lasts. Maintenance of electrolyte balance, not antibiotic treatment, is the cornerstone of treatment for campylobacter enteritis.Depending on the degree of dehydration, alternate measures may be taken including parenteral methods of hydration.[50][51] Indeed, most patients with this infection have a self-limited illness and do not require antibiotics at all; however they may be the best form of treatment in more severe cases of infection.
Antibiotic Treatment[edit]
Antibiotic treatment for Campylobacter infections is usually not required nor recommended. Antibiotics are limited for treating high-risk patients including immunocompromised and older individuals. Severe cases exhibiting symptoms such as bloody stools, fever, severe abdominal pain, pregnancy, infection with HIV, and prolonged illness (symptoms that last > 1 week) may also require treatment by antibiotics which can help to shorten the duration of the symptoms.[3][15][45] It is advisable to treat these infections with macrolide antibiotics, such as erythromycin or azithromycin. Erythromycin is inexpensive and limits toxic exposure to patients, however resistance rates are reportedly increasing; its use is continued however, as resistance rates remain below 5%.[52] Azithromycin usage is increasing due to various drug characteristics, including its once-a-day dosage, tolerability by patients, decreased relation to Infantile Hypertrophic Pyloric Stenosis (IHPS), and less negative symptoms; this is comparative to erythromycin. Fluoroquinolones are another source of treatment, however resistance rates of bacteria to this type of antibiotic is greatly increasing.[53]
Antibiotic resistance[edit]
Fluoroquinolone's were first approved as a treatment for Campylobacter infections in 1986, and were later U.S. Food and Drug Administration (FDA) approved in 1996 so as to control infections in poultry flocks. The CDC began monitoring Campylobacter in 1997 in the National Antimicrobial Resistance Monitoring System (NARMS). Data from NARMS indicated ciprofloxacin, a fluoroquinolone, had microbial resistance rates of 17% in 1997-1999, which further increased to 27% in 2015-1017.[53]
Prevention[edit]
Some simple food-handling practices can help prevent campylobacter infections.[1]
- Cook all poultry products thoroughly. Make sure that the meat is cooked throughout (no longer pink) and any juices run clear. All poultry should be cooked to reach a minimum internal temperature of 165 °F (74 °C).
- Wash hands with soap before preparing food.
- Wash hands with soap after handling raw foods of animal origin and before touching anything else.
- Prevent cross-contamination in the kitchen by using separate cutting boards for foods of animal origin and other foods and by thoroughly cleaning all cutting boards, countertops, and utensils with soap and hot water after preparing raw food of animal origin.
- Do not drink unpasteurized milk or untreated surface water.
- Make sure that people with diarrhea, especially children, wash their hands carefully and frequently with soap to reduce the risk of spreading the infection.
- Wash hands with soap after contact with pet feces.
Genome[edit]
The genome of C. jejuni strain NCTC11168 was published in 2000, revealing 1,641,481 base pairs (30.6% G+C) predicted to encode 1,654 proteins and 54 stable RNA species. The genome is unusual in that virtually no insertion sequences or phage-associated sequences and very few repeat sequences are found. One of the most striking findings in the genome was the presence of hypervariable sequences. These short homopolymeric runs of nucleotides were commonly found in genes encoding the biosynthesis or modification of surface structures, or in closely linked genes of unknown function. The apparently high rate of variation of these homopolymeric tracts may be important in the survival strategy of C. jejuni.[54] The genome was re-annotated in 2007 updating 18.2% of product functions.[55] Analysis also predicted the first pathogenicity island in C. jejuni amongst select strains, harbouring the bacteria's Type VI secretion system and putative cognate effectors.[56]
Initial transposon mutagenesis screens revealed 195 essential genes, although this number is likely to go up with additional analysis.[57]
Natural genetic transformation[edit]
C. jejuni is naturally competent for genetic transformation.[58] Natural genetic transformation is a sexual process involving DNA transfer from one bacterium to another through the intervening medium, and the integration of the donor sequence into the recipient genome by homologous recombination. C. jejuni freely takes up foreign DNA harboring genetic information responsible for antibiotic resistance.[58] Antibiotic resistance genes are more frequently transferred in biofilms than between planktonic cells (single cells that float in liquid media).[59]
Laboratory characteristics[edit]
| Characteristic | Result |
|---|---|
| Growth at 25 °C | − |
| Growth at 35–37 °C | + |
| Growth at 42 °C | + |
| Nitrate reduction | + |
| Catalase test | + |
| Oxidase test | + |
| Growth on MacConkey agar | + |
| Motility (wet mount) | + |
| Glucose utilization | − |
| Hippurate hydrolysis | + |
| Resistance to nalidixic acid | − |
| Resistance to cephalothin | + |

Under light microscopy, C. jejuni has a characteristic "sea-gull" shape as a consequence of its helical form. Campylobacter is grown on specially selective "CAMP" agar plates at 42 °C, the normal avian body temperature, rather than at 37 °C, the temperature at which most other pathogenic bacteria are grown. Since the colonies are oxidase positive, they usually only grow in scanty amounts on the plates. Microaerophilic conditions are required for luxurious growth. A selective blood agar medium (Skirrow's medium) can be used. Greater selectivity can be gained with an infusion of a cocktail of antibiotics: vancomycin, polymixin-B, trimethoprim, and actidione (Preston's agar),[60] and growth under microaerophilic conditions at 42 °C.
References[edit]
- ^ a b c "Campylobacter: Questions and Answers". U.S. Centers for Disease Control and Prevention. 2019-12-20. Retrieved 2020-01-02.
- ^ "Campylobacter". European Food Safety Authority. Retrieved 2 January 2020.
- ^ a b c d e f g h i Fischer GH, Hashmi MF, Paterek E (2024). "Campylobacter Infection". StatPearls. Treasure Island (FL): StatPearls Publishing. PMID 30725718. Retrieved 2024-02-29.
- ^ a b c "Campylobacter jejuni | Campylobacter Food Poisoning". www.about-campylobacter.com. Retrieved 2016-04-18.
- ^ Gundogdu O, Wren BW (March 2020). "Microbe Profile: Campylobacter jejuni - survival instincts". Microbiology. 166 (3): 230–232. doi:10.1099/mic.0.000906. PMID 32228803. S2CID 214750673.
- ^ Balaban M, Hendrixson DR (December 2011). "Polar flagellar biosynthesis and a regulator of flagellar number influence spatial parameters of cell division in Campylobacter jejuni". PLOS Pathogens. 7 (12): e1002420. doi:10.1371/journal.ppat.1002420. PMC 3228812. PMID 22144902.
- ^ Ryan KJ, Ray CG, eds. (2004). Sherris Medical Microbiology (4th ed.). McGraw Hill. ISBN 978-0-8385-8529-0.
- ^ "Online Bacteriological Analytical Manual, Chapter 7: Campylobacter". Food and Drug Administration.
- ^ Gorbach SL, Falagas M, eds. (2001). The 5 minute infectious diseases consult (1st ed.). Lippincott Williams & Wilkins. ISBN 978-0-683-30736-8."Multiple Campylobacter Genomes Sequenced". PLOS Biology. 3 (1): e40. 2005-01-04. doi:10.1371/journal.pbio.0030040. PMC 539341.
- ^ a b Perez-Perez GI, Blaser MJ (January 1996). Baronm S (ed.). Campylobacter and Helicobacter (4th ed.). Galveston (TX ): University of Texas Medical Branch at Galveston. ISBN 978-0-9631172-1-2. PMID 21413331.
- ^ Crushell E, Harty S, Sharif F, Bourke B (January 2004). "Enteric campylobacter: purging its secrets?". Pediatric Research. 55 (1): 3–12. doi:10.1203/01.PDR.0000099794.06260.71. PMID 14605259.
- ^ Fujimoto S, Amako K (June 1990). "Guillain-Barré syndrome and Campylobacter jejuni infection". Lancet. 335 (8701): 1350. doi:10.1016/0140-6736(90)91234-2. PMID 1971411. S2CID 38312888.
- ^ McCarthy N, Giesecke J (March 2001). "Incidence of Guillain-Barré syndrome following infection with Campylobacter jejuni". American Journal of Epidemiology. 153 (6): 610–614. doi:10.1093/aje/153.6.610. PMID 11257070.
- ^ a b "What is Reactive Arthritis?". Reactive Arthritis. 2019-02-06.
- ^ a b c Allos BM (April 2001). "Campylobacter jejuni Infections: update on emerging issues and trends". Clinical Infectious Diseases. 32 (8): 1201–1206. doi:10.1086/319760. PMID 11283810.
- ^ a b c d e Epps SV, Harvey RB, Hume ME, Phillips TD, Anderson RC, Nisbet DJ (November 2013). "Foodborne Campylobacter: infections, metabolism, pathogenesis and reservoirs". International Journal of Environmental Research and Public Health. 10 (12): 6292–6304. doi:10.3390/ijerph10126292. PMC 3881114. PMID 24287853.
- ^ a b Altekruse SF, Stern NJ, Fields PI, Swerdlow DL (1999). "Campylobacter jejuni--an emerging foodborne pathogen". Emerging Infectious Diseases. 5 (1): 28–35. doi:10.3201/eid0501.990104. PMC 2627687. PMID 10081669.
- ^ a b c Tegtmeyer N, Sharafutdinov I, Harrer A, Soltan Esmaeili D, Linz B, Backert S (2021). "Campylobacter Virulence Factors and Molecular Host-Pathogen Interactions". In Backert S (ed.). Current Topics in Microbiology and Immunology. Vol. 431. Cham: Springer International Publishing. pp. 169–202. doi:10.1007/978-3-030-65481-8_7. ISBN 978-3-030-65480-1. PMID 33620652.
- ^ a b c d e f g h Stahl M, Butcher J, Stintzi A (2012). "Nutrient acquisition and metabolism by Campylobacter jejuni". Frontiers in Cellular and Infection Microbiology. 2: 5. doi:10.3389/fcimb.2012.00005. PMC 3417520. PMID 22919597.
- ^ a b c d e f g Gao B, Vorwerk H, Huber C, Lara-Tejero M, Mohr J, Goodman AL, et al. (May 2017). Waldor M (ed.). "Metabolic and fitness determinants for in vitro growth and intestinal colonization of the bacterial pathogen Campylobacter jejuni". PLOS Biology. 15 (5): e2001390. doi:10.1371/journal.pbio.2001390. PMC 5438104. PMID 28542173.
- ^ Sellars MJ, Hall SJ, Kelly DJ (August 2002). "Growth of Campylobacter jejuni supported by respiration of fumarate, nitrate, nitrite, trimethylamine-N-oxide, or dimethyl sulfoxide requires oxygen". Journal of Bacteriology. 184 (15): 4187–4196. doi:10.1128/JB.184.15.4187-4196.2002. PMC 135223. PMID 12107136.
- ^ a b c d e f Hofreuter D (2014). "Defining the metabolic requirements for the growth and colonization capacity of Campylobacter jejuni". Frontiers in Cellular and Infection Microbiology. 4: 137. doi:10.3389/fcimb.2014.00137. PMC 4178425. PMID 25325018.
- ^ a b "Campylobacter: Questions and Answers". U.S. Centers for Disease Control and Prevention. 2019-12-20. Retrieved 2020-01-02.
- ^ a b c d e f g h i j k l m n o p q r s t u v w Kemper L, Hensel A (May 2023). "Campylobacter jejuni: targeting host cells, adhesion, invasion, and survival". Applied Microbiology and Biotechnology. 107 (9): 2725–2754. doi:10.1007/s00253-023-12456-w. PMC 10027602. PMID 36941439.
- ^ Kaakoush NO, Castaño-Rodríguez N, Mitchell HM, Man SM (July 2015). "Global Epidemiology of Campylobacter Infection". Clinical Microbiology Reviews. 28 (3): 687–720. doi:10.1128/CMR.00006-15. PMC 4462680. PMID 26062576.
- ^ Wallis MR (March 1994). "The pathogenesis of Campylobacter jejuni". British Journal of Biomedical Science. 51 (1): 57–64. PMID 7841837.
- ^ Korneev KV, Kondakova AN, Sviriaeva EN, Mitkin NA, Palmigiano A, Kruglov AA, et al. (2018). "Hypoacylated LPS from Foodborne Pathogen Campylobacter jejuni Induces Moderate TLR4-Mediated Inflammatory Response in Murine Macrophages". Frontiers in Cellular and Infection Microbiology. 8: 58. doi:10.3389/fcimb.2018.00058. PMC 5835049. PMID 29535976.
- ^ El Abbar FM, Li J, Owen HC, Daugherty CL, Fulmer CA, Bogacz M, et al. (2019-08-07). "RNA Binding by the Campylobacter jejuni Post-transcriptional Regulator CsrA". Frontiers in Microbiology. 10: 1776. doi:10.3389/fmicb.2019.01776. PMC 6692469. PMID 31447808.
- ^ Dasti JI, Tareen AM, Lugert R, Zautner AE, Gross U (April 2010). "Campylobacter jejuni: a brief overview on pathogenicity-associated factors and disease-mediating mechanisms". International Journal of Medical Microbiology. 300 (4): 205–211. doi:10.1016/j.ijmm.2009.07.002. PMID 19665925.
- ^ a b c d e f g h i j Gourley CR, Negretti NM, Konkel ME (October 2017). "The food-borne pathogen Campylobacter jejuni depends on the AddAB DNA repair system to defend against bile in the intestinal environment". Scientific Reports. 7 (1): 14777. Bibcode:2017NatSR...714777G. doi:10.1038/s41598-017-14646-9. PMC 5665897. PMID 29089630.
- ^ Alrubaye B, Abraha M, Almansour A, Bansal M, Wang H, Kwon YM, et al. (2019). "Microbial metabolite deoxycholic acid shapes microbiota against Campylobacter jejuni chicken colonization". PLOS ONE. 14 (7): e0214705. Bibcode:2019PLoSO..1414705A. doi:10.1371/journal.pone.0214705. PMC 6611565. PMID 31276498.
- ^ Negretti NM, Gourley CR, Clair G, Adkins JN, Konkel ME (November 2017). "The food-borne pathogen Campylobacter jejuni responds to the bile salt deoxycholate with countermeasures to reactive oxygen species". Scientific Reports. 7 (1): 15455. Bibcode:2017NatSR...715455N. doi:10.1038/s41598-017-15379-5. PMC 5684402. PMID 29133896.
- ^ Smith GR (June 2012). "How RecBCD enzyme and Chi promote DNA break repair and recombination: a molecular biologist's view". Microbiology and Molecular Biology Reviews. 76 (2): 217–228. doi:10.1128/MMBR.05026-11. PMC 3372252. PMID 22688812.
- ^ Sidor K, Skirecki T (May 2023). "A Bittersweet Kiss of Gram-Negative Bacteria: The Role of ADP-Heptose in the Pathogenesis of Infection". Microorganisms. 11 (5): 1316. doi:10.3390/microorganisms11051316. PMC 10221265. PMID 37317291.
- ^ Colles FM, McCarthy ND, Howe JC, Devereux CL, Gosler AG, Maiden MC (January 2009). "Dynamics of Campylobacter colonization of a natural host, Sturnus vulgaris (European starling)". Environmental Microbiology. 11 (1): 258–267. Bibcode:2009EnvMi..11..258C. doi:10.1111/j.1462-2920.2008.01773.x. PMC 2702492. PMID 18826435.
- ^ a b Rosenquist H, Sommer HM, Nielsen NL, Christensen BB (April 2006). "The effect of slaughter operations on the contamination of chicken carcasses with thermotolerant Campylobacter". International Journal of Food Microbiology. 108 (2): 226–232. doi:10.1016/j.ijfoodmicro.2005.12.007. PMID 16478636.
- ^ Oosterom J, DE Wilde GJ, DE Boer E, DE Blaauw LH, Karman H (August 1983). "Survival of Campylobacter jejuni during Poultry Processing and Pig Slaughtering". Journal of Food Protection. 46 (8): 702–706. doi:10.4315/0362-028X-46.8.702. PMID 30921893.
- ^ Ijaz UZ, Sivaloganathan L, McKenna A, Richmond A, Kelly C, Linton M, et al. (October 2018). "Comprehensive Longitudinal Microbiome Analysis of the Chicken Cecum Reveals a Shift From Competitive to Environmental Drivers and a Window of Opportunity for Campylobacter". Frontiers in Microbiology. 9: 2452. doi:10.3389/fmicb.2018.02452. PMC 6196313. PMID 30374341.
- ^ McKenna A, Ijaz UZ, Kelly C, Linton M, Sloan WT, Green BD, et al. (September 2020). "Impact of industrial production system parameters on chicken microbiomes: mechanisms to improve performance and reduce Campylobacter". Microbiome. 8 (1): 128. doi:10.1186/s40168-020-00908-8. PMC 7488076. PMID 32907634.
- ^ Center for Food Safety and Applied Nutrition. "Bad Bug Book - BBB - Campylobacter jejuni". www.fda.gov. Retrieved 2016-04-18.
- ^ Fonseca BB, Beletti ME, de Melo RT, Mendonça EP, Coelho LR, Nalevaiko PC, et al. (2014). "Campylobacter jejuni in commercial eggs". Brazilian Journal of Microbiology. 45 (1): 76–79. doi:10.1590/S1517-83822014000100011. PMC 4059329. PMID 24948916.
- ^ Skirrow MB, Jones DM, Sutcliffe E, Benjamin J (June 1993). "Campylobacter bacteraemia in England and Wales, 1981-91". Epidemiology and Infection. 110 (3): 567–573. doi:10.1017/s0950268800050986. PMC 2272297. PMID 8519321.
- ^ Allos BM (April 2001). "Campylobacter jejuni Infections: update on emerging issues and trends". Clinical Infectious Diseases. 32 (8): 1201–1206. doi:10.1086/319760. PMID 11283810.
- ^ Bonheur JL (2018-07-24). Anand BS (ed.). "Bacterial Gastroenteritis". Medscape Reference.
- ^ a b c Foodsafety.gov. "Campylobacter". www.foodsafety.gov. Retrieved 2016-04-18.
- ^ a b c d Javid MH, Ahmed SH (2019-02-02). Talavera F, Bronze MS (eds.). "Campylobacter Infections: Background, Pathophysiology, Epidemiology". Medscape.
- ^ a b "Diagnosis of a Campylobacter Infection". Marler Clark. Retrieved 2024-04-09.
- ^ Davis L, DiRita V (August 2008). "Growth and laboratory maintenance of Campylobacter jejuni". Current Protocols in Microbiology. Chapter 8: Unit 8A.1.1–8A.1.7. doi:10.1002/9780471729259.mc08a01s10. hdl:2027.42/154622. PMID 18729058.
- ^ Platts-Mills JA, Liu J, Gratz J, Mduma E, Amour C, Swai N, et al. (April 2014). Diekema DJ (ed.). "Detection of Campylobacter in stool and determination of significance by culture, enzyme immunoassay, and PCR in developing countries". Journal of Clinical Microbiology. 52 (4): 1074–1080. doi:10.1128/JCM.02935-13. PMC 3993515. PMID 24452175.
- ^ Fischer GH, Hashmi MF, Paterek E (2024), "Campylobacter Infection", StatPearls, Treasure Island (FL): StatPearls Publishing, PMID 30725718, retrieved 2024-02-29
- ^ "Antibiotic Resistance | Campylobacter | CDC". www.cdc.gov. 2022-06-27. Retrieved 2024-04-11.
- ^ Eiland LS, Jenkins LS (Jul–Sep 2008). "Optimal Treatment of Campylobacter Dysentery". The Journal of Pediatric Pharmacology and Therapeutics : JPPT. 13 (3): 170–174. doi:10.5863/1551-6776-13.3.170. PMC 3462042. PMID 23055879.
- ^ a b Eiland LS, Jenkins LS (Jul–Sep 2008). "Optimal Treatment of Campylobacter Dysentery". The Journal of Pediatric Pharmacology and Therapeutics : JPPT : The Official Journal of Ppag. 13 (3): 170–174. doi:10.5863/1551-6776-13.3.170. PMC 3462042. PMID 23055879.
- ^ Parkhill J, Wren BW, Mungall K, Ketley JM, Churcher C, Basham D, et al. (February 2000). "The genome sequence of the food-borne pathogen Campylobacter jejuni reveals hypervariable sequences". Nature. 403 (6770): 665–668. Bibcode:2000Natur.403..665P. doi:10.1038/35001088. PMID 10688204.
- ^ Gundogdu O, Bentley SD, Holden MT, Parkhill J, Dorrell N, Wren BW (June 2007). "Re-annotation and re-analysis of the Campylobacter jejuni NCTC11168 genome sequence". BMC Genomics. 8 (1): 162. doi:10.1186/1471-2164-8-162. PMC 1899501. PMID 17565669.
- ^ Robinson L, Liaw J, Omole Z, Xia D, van Vliet AH, Corcionivoschi N, et al. (29 June 2021). "Bioinformatic Analysis of the Campylobacter jejuni Type VI Secretion System and Effector Prediction". Frontiers in Microbiology. 12: 694824. doi:10.3389/fmicb.2021.694824. PMC 8285248. PMID 34276628.
- ^ Stahl M, Stintzi A (June 2011). "Identification of essential genes in C. jejuni genome highlights hyper-variable plasticity regions". Functional & Integrative Genomics. 11 (2): 241–257. doi:10.1007/s10142-011-0214-7. PMID 21344305. S2CID 24054117.
- ^ a b Bae J, Oh E, Jeon B (December 2014). "Enhanced transmission of antibiotic resistance in Campylobacter jejuni biofilms by natural transformation". Antimicrobial Agents and Chemotherapy. 58 (12): 7573–7575. doi:10.1128/AAC.04066-14. PMC 4249540. PMID 25267685.
- ^ Michaelis C, Grohmann E (February 2023). "Horizontal Gene Transfer of Antibiotic Resistance Genes in Biofilms". Antibiotics. 12 (2): 328. doi:10.3390/antibiotics12020328. PMC 9952180. PMID 36830238.
- ^ Bolton FJ, Robertson L (April 1982). "A selective medium for isolating Campylobacter jejuni/coli". Journal of Clinical Pathology. 35 (4): 462–467. doi:10.1136/jcp.35.4.462. PMC 497682. PMID 7042765.
External links[edit]
- Campylobacter jejuni genomes and related information at PATRIC, a Bioinformatics Resource Center funded by NIAID
- Current research on Campylobacter jejuni at the Norwich Research Park Archived 2017-06-14 at the Wayback Machine
- Type strain of Campylobacter jejuni at BacDive - the Bacterial Diversity Metadatabase