 | |
| Clinical data | |
|---|---|
| Trade names | Mydriacyl, others |
| AHFS/Drugs.com | Monograph |
| License data |
|
| Pregnancy category |
|
| Routes of administration | Topical eye drops |
| ATC code | |
| Legal status | |
| Legal status | |
| Pharmacokinetic data | |
| Protein binding | 45% |
| Identifiers | |
| |
| CAS Number | |
| PubChem CID | |
| IUPHAR/BPS | |
| DrugBank | |
| ChemSpider | |
| UNII | |
| KEGG | |
| ChEMBL | |
| CompTox Dashboard (EPA) | |
| ECHA InfoCard | 100.014.673 |
| Chemical and physical data | |
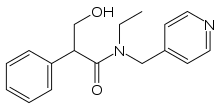
| Formula | C17H20N2O2 |
| Molar mass | 284.359 g·mol−1 |
| 3D model (JSmol) | |
| |
| |
| | |
Tropicamide, sold under the brand name Mydriacyl among others, is a medication used to dilate the pupil and help with examination of the eye.[3] Specifically it is used to help examine the back of the eye.[4] It is applied as eye drops.[3] Effects occur within 40 minutes and last for up to a day.[3]
Common side effects include blurry vision, increased intraocular pressure, and sensitivity to light.[3] Another rare but severe side effect is psychosis, particularly in children.[3] It is unclear if use during pregnancy is safe for the fetus.[5] Tropicamide is in the antimuscarinic part of the anticholinergic family of medications.[3] It works by making the muscles within the eye unable to respond to nerve signals.[3]
Tropicamide was approved for medical use in the United States in 1960.[3] It is on the World Health Organization's List of Essential Medicines.[6]
Medical use[edit]

Tropicamide is an antimuscarinic drug that produces short acting mydriasis (dilation of the pupil) and cycloplegia[7] when applied as eye drops. It is used to allow better examination of the lens, vitreous humor, and retina. Due to its relatively short duration of effect (4–8 hours), it is typically used during eye examinations such as the dilated fundus examination, but it may also be used before or after eye surgery. Cycloplegic drops are often also used to treat anterior uveitis, decreasing risk of posterior synechiae and decreasing inflammation in the anterior chamber of the eye.
Tropicamide is occasionally administered in combination with p-hydroxyamphetamine (brand name Paremyd), which is a sympathomimetic. The use of the sympathomimetic drug causes the iris dilator muscle to be directly stimulated, causing increased dilation. In the United States, the sympathomimetic drop most commonly used along with tropicamide, is 2.5% phenylephrine hydrochloride (brand name AK-Dilate).
Side effects[edit]
Tropicamide induces transient stinging and a slight and transient rise in intraocular pressure in the majority of patients. It may cause redness or conjunctivitis (inflammation) and also blurs near vision for a short while after instillation (care must be taken, and the patient must only drive when vision returns to normal). Tropicamide may, in very rare cases,[8] cause an attack of acute angle-closure glaucoma. This tends to be in patients with narrow anterior chamber angles, and closure risk must be assessed by the practitioner prior to instillation.
Tropicamide is often preferred to atropine because atropine has a longer half-life, causing prolonged dilation and blurry vision for up to a week. Atropine has less sting effect, but can be toxic or fatal if ingested in large quantities by children or adults.
With eye drops, systemic effects are minimal to nonexistent due to very low absorption into the bloodstream.[9]
Recreational use[edit]
Tropicamide is sometimes abused (injected intravenously e.g. by insulin syringe) as an inexpensive recreational deliriant drug (along with naphazoline). This was initially reported in Russia, but has subsequently spread to various other countries in the former Soviet Union and around Europe, and later in the United States.[10][11][12][13]
Stereochemistry[edit]
Tropicamide has a chiral center and two enantiomers. Medications are racemates.[14]
| Enantiomers | |
|---|---|
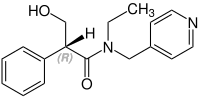 (R)-Tropicamid CAS: 92934-63-9 |
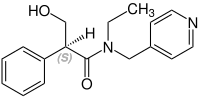 (S)-Tropicamid CAS: 92934-64-0 |
References[edit]
- ^ "Summary for ARTG Entry: 25356 Mydriacyl tropicamide 0.5% eye drops bottle". Therapeutic Goods Administration. Archived from the original on 5 June 2023. Retrieved 5 June 2023.
- ^ "Mydriacyl 1% eye drops, solution - Summary of Product Characteristics (SmPC)". Electronic Medicines Compendium. 12 February 2020. Archived from the original on 27 June 2022. Retrieved 29 July 2020.
- ^ a b c d e f g h "Tropicamide". Drugs.com. American Society of Health-System Pharmacists. Archived from the original on 28 December 2016. Retrieved 8 December 2016.
- ^ World Health Organization (2009). Stuart MC, Kouimtzi M, Hill SR (eds.). WHO Model Formulary 2008. World Health Organization. p. 314. hdl:10665/44053. ISBN 9789241547659.
- ^ "Tropicamide ophthalmic Use During Pregnancy". Drugs.com. Archived from the original on 28 December 2016. Retrieved 28 December 2016.
- ^ World Health Organization (2019). World Health Organization model list of essential medicines: 21st list 2019. Geneva: World Health Organization. hdl:10665/325771. WHO/MVP/EMP/IAU/2019.06. License: CC BY-NC-SA 3.0 IGO.
- ^ Manny RE, Hussein M, Scheiman M, Kurtz D, Niemann K, Zinzer K (July 2001). "Tropicamide (1%): an effective cycloplegic agent for myopic children". Investigative Ophthalmology & Visual Science. 42 (8): 1728–35. PMID 11431435. Archived from the original on 2013-01-12.
- ^ Liew G, Mitchell P, Wang JJ, Wong TY (January 2006). "Fundoscopy: to dilate or not to dilate?". BMJ. 332 (7532): 3. doi:10.1136/bmj.332.7532.3. PMC 1325111. PMID 16399709.
- ^ Vuori ML, Kaila T, Iisalo E, Saari KM (1994-01-01). "Systemic absorption and anticholinergic activity of topically applied tropicamide". Journal of Ocular Pharmacology. 10 (2): 431–437. doi:10.1089/jop.1994.10.431. PMID 8083562.
- ^ Bersani FS, Corazza O, Simonato P, Mylokosta A, Levari E, Lovaste R, Schifano F (2013). "Drops of madness? Recreational misuse of tropicamide collyrium; early warning alerts from Russia and Italy". General Hospital Psychiatry. 35 (5): 571–3. doi:10.1016/j.genhosppsych.2013.04.013. PMID 23706777.
- ^ Walker S (21 June 2011). "Krokodil: The drug that eats junkies". The Independent. Archived from the original on 2017-09-22.
- ^ Bersani FS, Imperatori C, Prilutskaya M, Kuliev R, Corazza O (July 2015). "Injecting eye-drops: a mini-review on the non-clinical use of tropicamide". Hum Psychopharmacol. 30 (4): 262–4. doi:10.1002/hup.2481. PMID 26216560. S2CID 190289.
- ^ Bellman V, Ukolova A, Erovichenkova E, Lam S, Srivastava HK, Bruce J, Burgess DM (November 2022). "Abuse of tropicamide eye drops: review of clinical data". Braz J Psychiatry. 44 (5): 522–531. doi:10.47626/1516-4446-2021-2446. PMC 9561840. PMID 35739063.
- ^ Rote Liste (in German). Vol. 57. Frankfurt/Main: Rote Liste Service GmbH. 2017. p. 224. ISBN 978-3-946057-10-9.
Arzneimittelverzeichnis für Deutschland (einschließlich EU-Zulassungen und bestimmter Medizinprodukte)
External links[edit]
- "Tropicamide". Drug Information Portal. U.S. National Library of Medicine.