| |
| |
| Names | |
|---|---|
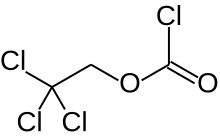
| Preferred IUPAC name
2,2,2-Trichloroethyl carbonochloridate | |
| Other names
2,2,2-Trichlorethoxycarbonyl chloride
Trichloroethyl chloroformate | |
| Identifiers | |
3D model (JSmol)
|
|
| ChemSpider | |
| ECHA InfoCard | 100.037.587 |
| EC Number |
|
PubChem CID
|
|
| UNII | |
CompTox Dashboard (EPA)
|
|
| |
| |
| Properties | |
| C3H2Cl4O2 | |
| Molar mass | 211.85 g·mol−1 |
| Density | 1.539 g/cm3 |
| Melting point | 0 °C (32 °F; 273 K) |
| Boiling point | 171 to 172 °C (340 to 342 °F; 444 to 445 K) |
| Hazards | |
| GHS labelling: | |
  
| |
| Danger | |
| H302, H314, H330, H331 | |
| P260, P261, P264, P270, P271, P280, P284, P301+P312, P301+P330+P331, P303+P361+P353, P304+P340, P305+P351+P338, P310, P311, P320, P321, P330, P363, P403+P233, P405, P501 | |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa).
| |
Trichloroethyl chloroformate is used in organic synthesis for the introduction of the trichloroethyl chloroformate (Troc) protecting group for amines, thiols and alcohols. It readily cleaves vs other carbamates and can be used in an overall protecting group strategy.
The troc group is traditionally removed via Zn insertion in the presence of acetic acid, resulting in elimination and decarboxylation.
Amine protection – 2,2,2-Trichloroethoxycarbonyl (Troc)[edit]
2,2,2-Trichloroethoxycarbonyl (Troc) group is largely used as a protecting group for amines in organic synthesis.
Most common amine protection methods[edit]
- 2,2,2-Trichloroethyl chloroformate, pyridine or aqueous sodium hydroxide at ambient temperature[2]
References[edit]
- ^ 2,2,2-Trichloroethyl chloroformate Archived 2012-09-09 at archive.today at Sigma-Aldrich
- ^ Marullo, N. P.; Wagener, E. H. (1969-01-01). "Structural organic chemistry by nmr. III. Isomerization of compounds containing the carbon-nitrogen double bond". Tetrahedron Letters. 10 (30): 2555–2558. doi:10.1016/S0040-4039(01)88566-X.



