 | |
| Clinical data | |
|---|---|
| Trade names | Uptravi |
| Other names | ACT-293987, NS-304 |
| License data | |
| Routes of administration | By mouth, intravenous |
| Drug class | prostacyclin receptor agonist |
| ATC code | |
| Legal status | |
| Legal status | |
| Pharmacokinetic data | |
| Bioavailability | 49% |
| Protein binding | 99% |
| Metabolism | Activation by carboxylesterases, inactivation by CYP2C8 and others |
| Metabolites | ACT-333679, the free acid (active metabolite) |
| Elimination half-life | 0.8–2.5 h (selexipag) and 6.2–13.5 h (ACT-333679) |
| Excretion | 93% faeces |
| Identifiers | |
| |
| CAS Number | |
| PubChem CID | |
| IUPHAR/BPS | |
| DrugBank | |
| ChemSpider | |
| UNII | |
| KEGG | |
| ChEBI | |
| ChEMBL | |
| ECHA InfoCard | 100.237.916 |
| Chemical and physical data | |
| Formula | C26H32N4O4S |
| Molar mass | 496.63 g·mol−1 |
| 3D model (JSmol) | |
| |
| |
| | |
Selexipag, sold under the brand name Uptravi, is a medication developed by Actelion for the treatment of pulmonary arterial hypertension (PAH).[3][4] Selexipag and its active metabolite, ACT-333679 (or MRE-269, the free carboxylic acid), are agonists of the prostacyclin receptor, which leads to vasodilation in the pulmonary circulation.[5] It is taken by mouth or administered intravenously.[3][6]
The most common side effects include headache, diarrhea, nausea and vomiting, jaw pain, myalgia (muscle pain), pain in the limbs, arthralgia (joint pain) and flushing.[4]
It is available as a generic medication.[7]
Medical uses[edit]
Selexipag is indicated for the treatment of pulmonary arterial hypertension.[3][4]
Contraindications[edit]
In the European Union, use of selexipag together with strong inhibitors of the liver enzyme CYP2C8, such as gemfibrozil, is contraindicated because it increases concentrations of selexipag twofold, and its active metabolite 11-fold, potentially leading to more adverse effects.[4][8]
Adverse effects[edit]
The adverse effects of selexipag are similar to those of intravenous prostacyclins used for pulmonary arterial hypertension. Common side effects include headache and jaw pain. An increased risk for hyperthyroidism has also been noted in people taking selexipag.[9]
Pharmacology[edit]
Mechanism of action[edit]

Selexipag and its active metabolite ACT-333679 act on the prostacyclin receptor of lung tissue, with the latter being 37-fold more potent. They are selective for the prostacyclin receptor. Binding to this receptor leads to three major effects: increased vasodilation of the arteries, decreased cell proliferation and inhibition of platelet aggregation,[9] all beneficial in the treatment of pulmonary arterial hypertension.
Pharmacokinetics[edit]
Selexipag is quickly absorbed from the gut and hydrolyzed in the intestines and the liver to ACT-333679 by carboxylesterases. Absolute bioavailability is about 49%, most likely because of a high first-pass effect. Highest concentrations in the blood plasma are reached after one to three hours for selexipag and after three to four hours for the active metabolite. When in the circulation, about 99% of both substances are bound to plasma proteins, namely to albumin and alpha-1-acid glycoprotein to equal amounts.[9]
The liver enzymes CYP2C8 and, to a lesser extent, CYP3A4, hydroxylate and dealkylate the active substance, thereby inactivating it. Besides, ACT-333679 is glucuronidized by the enzymes UGT1A3 and UGT2B7. The terminal half-life of selexipag is 0.8 to 2.5 hours, that of the active metabolite is 6.2 to 13.5 hours.[9]
Chemistry[edit]
Synthesis[edit]
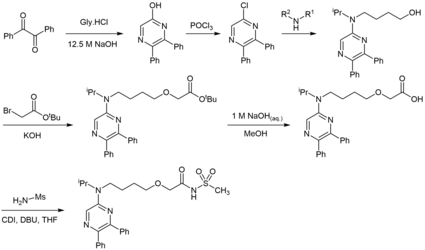
The synthesis of celexipag begins from two inexpensive compounds, glycine hydrochloride and benzil, condensed under basic conditions.[10][11]
History[edit]
The U.S. Food and Drug Administration (FDA) granted selexipag orphan drug designation for pulmonary arterial hypertension[12] and for the treatment of chronic thromboembolic pulmonary hypertension.[13] It was approved by the FDA in December 2015.[3][14]
In the European Union, the drug was approved in May 2016.[4][9]
Society and culture[edit]
Economics[edit]
The expected price for the drug in the US is $160,000 to $170,000 per patient before rebates.[15]
References[edit]
- ^ "Prescription medicines: registration of new chemical entities in Australia, 2016". Therapeutic Goods Administration (TGA). 21 June 2022. Archived from the original on 10 April 2023. Retrieved 10 April 2023.
- ^ "Health Canada New Drug Authorizations: 2016 Highlights". Health Canada. 14 March 2017. Retrieved 7 April 2024.
- ^ a b c d e "Uptravi- selexipag tablet, coated Uptravi Titration Pack- selexipag kit". DailyMed. Archived from the original on 30 July 2021. Retrieved 30 July 2021.
- ^ a b c d e f "Uptravi EPAR". European Medicines Agency. 1 July 2022. Archived from the original on 12 May 2021. Retrieved 27 August 2023. Text was copied from this source which is copyright European Medicines Agency. Reproduction is authorized provided the source is acknowledged.
- ^ Sitbon O, Morrell N (December 2012). "Pathways in pulmonary arterial hypertension: the future is here". European Respiratory Review. 21 (126): 321–327. doi:10.1183/09059180.00004812. PMC 9487224. PMID 23204120.
- ^ "Uptravi (selexipag) Receives FDA Approval for Intravenous Use in Adult Patients with Pulmonary Arterial Hypertension (PAH)". Janssen Pharmaceutical Companies (Press release). 30 July 2021. Retrieved 30 July 2021.
- ^ "Competitive Generic Therapy Approvals". U.S. Food and Drug Administration (FDA). 29 June 2023. Archived from the original on 29 June 2023. Retrieved 29 June 2023.
- ^ Information des Bundesamtes für Sicherheit im Gesundheitswesen zu Uptravi (in German), Österreichisches Bundesamt für Sicherheit im Gesundheitswesen, 7 June 2017
- ^ a b c d e "Uptravi: Authorisation details". European Medicines Agency. 12 May 2016. Archived from the original on 20 June 2018. Retrieved 8 June 2017.
- ^ Asaki T, Kuwano K, Morrison K, Gatfield J, Hamamoto T, Clozel M (September 2015). "Selexipag: An Oral and Selective IP Prostacyclin Receptor Agonist for the Treatment of Pulmonary Arterial Hypertension". Journal of Medicinal Chemistry. 58 (18): 7128–7137. doi:10.1021/acs.jmedchem.5b00698. PMID 26291199.
- ^ Flick AC, Ding HX, Leverett CA, Kyne RE, Liu KK, Fink SJ, et al. (August 2017). "Synthetic Approaches to the New Drugs Approved During 2015". Journal of Medicinal Chemistry. 60 (15): 6480–6515. doi:10.1021/acs.jmedchem.7b00010. PMID 28421763.
- ^ "Selexipag Orphan Drug Designations and Approvals". U.S. Food and Drug Administration (FDA). 1 January 2013. Archived from the original on 28 August 2023. Retrieved 27 August 2023.
- ^ "Selexipag Orphan Drug Designations and Approvals". U.S. Food and Drug Administration (FDA). 1 January 2013. Archived from the original on 28 August 2023. Retrieved 27 August 2023.
- ^ "Uptravi Tablets". U.S. Food and Drug Administration (FDA). 28 January 2016. Archived from the original on 28 August 2023. Retrieved 27 August 2023.
- ^ "Actelion sees Uptravi price of $160,000-170,000/patient". Reuters. 5 January 2016. Archived from the original on 1 November 2018. Retrieved 6 January 2016.
External links[edit]
- Clinical trial number NCT03187678 for "Safety Study of the Switch From Oral Selexipag to Intravenous Selexipag in Subjects With Stable Pulmonary Arterial Hypertension" at ClinicalTrials.gov