| SLC46A3 | |||||||||||||||||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Identifiers | |||||||||||||||||||||||||||||||||||||||||||||||||||
| Aliases | SLC46A3, FKSG16, SLC46A3 (gene), solute carrier family 46 member 3 | ||||||||||||||||||||||||||||||||||||||||||||||||||
| External IDs | OMIM: 616764 MGI: 1918956 HomoloGene: 41733 GeneCards: SLC46A3 | ||||||||||||||||||||||||||||||||||||||||||||||||||
| |||||||||||||||||||||||||||||||||||||||||||||||||||
| |||||||||||||||||||||||||||||||||||||||||||||||||||
| |||||||||||||||||||||||||||||||||||||||||||||||||||
| |||||||||||||||||||||||||||||||||||||||||||||||||||
| |||||||||||||||||||||||||||||||||||||||||||||||||||
| Wikidata | |||||||||||||||||||||||||||||||||||||||||||||||||||
| |||||||||||||||||||||||||||||||||||||||||||||||||||
Solute carrier family 46 member 3 (SLC46A3) is a protein that in humans is encoded by the SLC46A3 gene.[5] Also referred to as FKSG16, the protein belongs to the major facilitator superfamily (MFS) and SLC46A family.[6] Most commonly found in the plasma membrane and endoplasmic reticulum (ER), SLC46A3 is a multi-pass membrane protein with 11 α-helical transmembrane domains.[7][8] It is mainly involved in the transport of small molecules across the membrane through the substrate translocation pores featured in the MFS domain.[9][10] The protein is associated with breast and prostate cancer, hepatocellular carcinoma (HCC), papilloma, glioma, obesity, and SARS-CoV.[11][12][13][14][15][16] Based on the differential expression of SLC46A3 in antibody-drug conjugate (ADC)-resistant cells and certain cancer cells, current research is focused on the potential of SLC46A3 as a prognostic biomarker and therapeutic target for cancer.[17] While protein abundance is relatively low in humans, high expression has been detected particularly in the liver, small intestine, and kidney.[18][19]
Gene[edit]
The SLC46A3 gene, also known by its aliases solute carrier family 46 member 3 and FKSG16, is located at 13q12.3 on the reverse strand in humans.[5] The gene spans 18,950 bases from 28,700,064 to 28,719,013 (GRCh38/hg38), flanked by POMP upstream and CYP51A1P2 downstream.[6][20] SLC46A3 contains 6 exons and 5 introns.[5] There are two paralogs for this gene, SLC46A1 and SLC46A2, and orthologs as distant as fungi.[21] So far, more than 4580 single nucleotide polymorphisms (SNPs) for this gene have been identified.[22] SLC46A3 is expressed at relatively low levels, about 0.5x the average gene.[23] Gene expression is peculiarly high in the liver, small intestine, and kidney.[18][19]
Transcript[edit]
Transcript Variants[edit]
SLC46A3 has multiple transcript variants produced by different promoter regions and alternative splicing.[5][24] A total of 4 transcript variants are found in the RefSeq database.[25] Variant 1 is most abundant.[26]
| Transcript Variant | Accession Number | Length (bp) | Description |
|---|---|---|---|
| 1[26] | NM_181785.4 | 3302 | MANE select. Variant 1 encodes isoform a. |
| 2[27] | NM_001135919.2 | 2758 | Variant 2 encodes isoform b. It lacks a segment in the 3' coding region and the resulting frameshift causes isoform b to have a longer C-terminus than isoform a. |
| 3[28] | NM_001347960.1 | 3099 | Variant 3 also encodes isoform a. Variants 1 and 3 differ in their 5' untranslated regions (UTRs). |
| X1[29] | XM_005266361.2 | 1845 | Variant X1 encodes isoform X1. |
*Lengths shown do not include introns.
Protein[edit]
Isoforms[edit]
3 isoforms have been reported for SLC46A3.[5] Isoform a is MANE select and most abundant.[30] All isoforms contain the MFS and MFS_1 domains as well as the 11 transmembrane regions.[8][31][32]
| Isoform | Accession Number | Length (aa) | Transcript |
|---|---|---|---|
| a[30][8] | NP_861450.1 | 461 | 1,3 |
| b[31] | NP_001129391.1 | 463 | 2 |
| X1[32] | XP_005266418.1 | 463 | X1 |
*Lengths shown are for the precursor proteins.
Properties[edit]
SLC46A3 is an integral membrane protein 461 amino acids (aa) of length with a molecular weight (MW) of 51.5 kDa.[33] The basal isoelectric point (pI) for this protein is 5.56.[34] The protein contains 11 transmembrane domains in addition to domains MFS and MFS_1.[30] MFS and MFS_1 domains largely overlap and contain 42 putative substrate translocation pores that are predicted to bind substrates for transmembrane transport.[10] The substrate translocation pores have access to both sides of the membrane in an alternating fashion through a conformational change. SLC46A3 lacks charged and polar amino acids while containing an excess of nonpolar amino acids, particularly phenylalanine (Phe).[33] The resulting hydrophobicity is mostly concentrated in the transmembrane regions for interactions with the fatty acid chains in the lipid bilayer.[35] The transmembrane domains also have a shortage of proline (Pro), a helix breaker.[33]

The protein sequence contains mixed, positive, and negative charge clusters, one of each, which are high in glutamine (Glu).[33] The clusters are located outside the transmembrane regions, and thus are solvent-exposed. Two 0 runs that run through several transmembrane domains in addition to a +/* run in between two transmembrane domains are also present. The protein contains a C-(X)2-C motif (CLLC), which is mostly present in metal-binding proteins and oxidoreductases.[36] A sorting-signal sequence motif, YXXphi, is also found at Tyr246 - Phe249 (YMLF) and Tyr446 - Leu449 (YELL).[37][38] This Y-based sorting signal directs the trafficking within the endosomal and the secretory pathways of integral membrane proteins by interacting with the mu subunits of the adaptor protein (AP) complex.[39] The signal-transducing adaptor protein 1 (STAP1) Src homology 2 (SH2) domain binding motif at Tyr446 - Ile450 (YELLI) is a phosphotyrosine (pTyr) pocket that serves as a docking site for the SH2 domain, which is central to tyrosine kinase signaling.[37][40] Multiple periodicities typical of an α-helix (periods of 3.6 residues in the hydrophobicity) encompass transmembrane domains.[41] 3 tandem repeats with core block lengths of 3 aa (GNYT, VSTF, STFI) are observed throughout the sequence.[33]
Secondary Structure[edit]
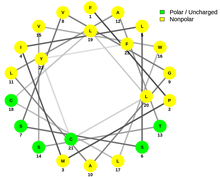
Based on results by Ali2D, the secondary structure of SLC46A3 is rich in α-helices with random coils in between.[42] More precisely, the protein is predicted to be composed of 62.9% α-helix, 33.8% random coil, and 3.3% extended strand. The regions of α-helices span the majority of the transmembrane domains. The signal peptide is also predicted to form an α-helix, most likely in the h-region.[43] The amphipathic α-helices possess a particular orientation with charged/polar and nonpolar residues on opposite sides of the helix mainly due to the hydrophobic effect.[44]
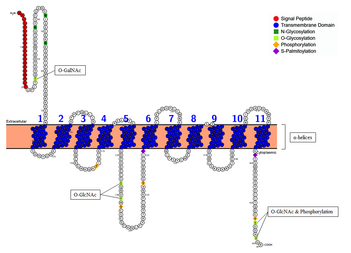
Membrane topology of SLC46A3 shows the 11 α-helical transmembrane domains embedded in the membrane with the N-terminus oriented toward the extracellular region (or lumen of the ER) and the C-terminus extended to the cytoplasmic region.[45][46]
Tertiary Structure[edit]
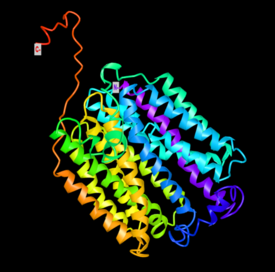
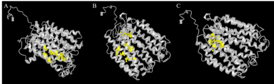
Model for the tertiary structure of SLC46A3 was constructed by I-TASSER based on a homologous crystal structure of the human organic anion transporter MFSD10 (Tetran) with a TM-score of 0.853.[47][48][49] The structure contains a cluster of 17 α-helices that spans the membrane and random coils that connect those α-helices. Multiple ligand binding sites are also predicted to reside in the structure, including those for (2S)-2,3-dihydroxypropyl(7Z)-pentadec-7-enoate (78M), cholesterol hemisuccinate (Y01), and octyl glucose neopentyl glycol (37X).[50][51]
| Ligand | C-score | Cluster Size | Ligand Binding Site Residues |
|---|---|---|---|
| 78M | 0.05 | 3 | 112, 116, 197, 198, 201, 204, 208 |
| Y01 | 0.05 | 3 | 89, 241, 265, 269, 273, 391, 394, 399 |
| 37X | 0.03 | 2 | 86, 89, 90, 94, 109, 136 |
Regulation of Gene Expression[edit]
Gene Level Regulation[edit]
Promoter[edit]
SLC46A3 carries 4 promoter regions that lead to different transcript variants as identified by ElDorado at Genomatix.[24] Promoter A supports transcript variant 1 (GXT_2836199).
| Promoter | Name | Start | End | Length (bp) | Transcript |
|---|---|---|---|---|---|
| A | GXP_190678 | 28718802 | 28720092 | 1291 | GXT_2775378, GXT_29165870, GXT_23385588, GXT_2836199, GXT_26222267, GXT_22739111, GXT_23500299 |
| B | GXP_190676 | 28714934 | 28715973 | 1040 | GXT_2785139 |
| C | GXP_190679 | 28713272 | 28714311 | 1040 | GXT_2781051 |
| D | GXP_19677 | 28704518 | 28705557 | 1040 | GXT_2781071 |
*The coordinates are for GRCh38.
Transcription Factors[edit]
Transcription factors (TFs) bind to the promoter region of SLC46A3 and modulate the transcription of the gene.[52] The table below shows a curated list of predicted TFs. MYC proto-oncogene (c-Myc), the strongest hit at Genomatix with a matrix similarity of 0.994, dimerizes with myc-associated factor X (MAX) to affect gene expression in a way that increases cell proliferation and cell metabolism.[53][54] Its expression is highly amplified in the majority of human cancers, including Burkitt's lymphoma. The heterodimer can repress gene expression by binding to myc-interacting zinc finger protein 1 (MIZ1), which also binds to the promoter of SLC46A3. CCAAT-displacement protein (CDP) and nuclear transcription factor Y (NF-Y) have multiple binding sites within the promoter sequence (3 sites for CDP and 2 sites for NF-Y).[53] CDP, also known as Cux1, is a transcriptional repressor.[55] NF-Y is a heterotrimeric complex of three different subunits (NF-YA, NF-YB, NF-YC) that regulates gene expression, both positively and negatively, by binding to the CCAAT box.[56]
| Transcription Factor | Description | Matrix Similarity |
|---|---|---|
| HIF | hypoxia inducible factor | 0.989 |
| c-Myc | myelocytomatosis oncogene (c-Myc proto-oncogene) | 0.994 |
| GATA1 | GATA-binding factor 1 | 0.983 |
| PXR/RXR | pregnane X receptor / retinoid X receptor heterodimer | 0.833 |
| RREB1 | Ras-responsive element binding protein 1 | 0.815 |
| TFCP2L1 | transcription factor CP2-like 1 (LBP-9) | 0.897 |
| ZNF34 | zinc finger protein 34 (KOX32) | 0.852 |
| MIZ1 | myc-interacting zinc finger protein 1 (ZBTB17) | 0.962 |
| RFX5 | regulatory factor X5 | 0.758 |
| CEBPB | CCAAT/enhancer-binding protein beta | 0.959 |
| KLF2 | Kruppel-like factor 2 (LKLF) | 0.986 |
| CSRNP1 | cysteine/serine-rich nuclear protein 1 (AXUD1) | 1.000 |
| CDP | CCAAT-displacement protein (CDP/Cux) | 0.983
0.949 0.955 |
| NF-Y | nuclear transcription factor Y | 0.944
0.934 |
| ZNF692 | zinc finger protein 692 | 0.855 |
| KAISO | transcription factor Kaiso (ZBTB33) | 0.991 |
| SP4 | transcription factor Sp4 | 0.908 |
| ZBTB24 | zinc finger and BTB domain containing 24 | 0.864 |
| E2F4 | E2F transcription factor 4 | 0.982 |
Expression Pattern[edit]

RNAseq data show SLC46A3 most highly expressed in the liver, small intestine, and kidney and relatively low expression in the brain, skeletal muscle, salivary gland, placenta, and stomach.[18][19][57] In fetuses of 10 – 20 weeks, the adrenal gland and intestine report high expression while the heart, kidney, lung, and stomach demonstrate the opposite.[58] Microarray data from NCBI GEO present high expression in pancreatic islets, pituitary gland, lymph nodes, peripheral blood, and liver with percentile ranks of 75 or above.[59] Conversely, tissues among the most lowly expressed levels of SLC46A3 include bronchial epithelial cells, caudate nucleus, superior cervical ganglion, smooth muscle, and colorectal adenocarcinoma, all with percentile ranks below 15. Immunohistochemistry supports expression of the gene in the liver and kidney, as well as in skin tissues, while immunoblotting (western blotting) provides evidence for protein abundance in the liver and tonsils, in addition to in papilloma and glioma cells.[14]
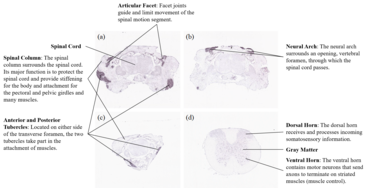
In situ hybridization data show ubiquitous expression of the gene in mouse embryos at stage E14.5 and the adult mouse brain at postnatal days 56 (P56).[60][61] In the spinal column of juvenile mouse (P4), SLC46A3 is relatively highly expressed in the articular facet, neural arch, and anterior and posterior tubercles.[62] The dorsal horn shows considerable expression in the cervical spine of adult mouse (P56).[63]
Transcript Level Regulation[edit]
RNA-binding Proteins[edit]
RNA-binding proteins (RBPs) that bind to the 5' or 3' UTR regulate mRNA expression by getting involved in RNA processing and modification, nuclear export, localization, and translation.[64] A list of some of the most highly predicted RBPs in conserved regions of the 5' and 3' UTRs are shown below.
| Protein | Description | Motif | P-value |
|---|---|---|---|
| MBNL1 (muscleblind like splicing regulator 1) | modulates alternative splicing of pre-mRNAs; binds specifically to expanded dsCUG RNA with unusual size CUG repeats; contributes to myotonic dystrophy | ygcuky | 8.38×10−3
2.52×10−3 |
| ZC3H10 (zinc finger CCCH-type containing 10) | functions as a tumor suppressor by inhibiting the anchorage-independent growth of tumor cells; mitochondrial regulator | ssagcgm | 6.33×10−3 |
| FXR2 (FMR1 autosomal homolog 2) | associated with the 60S large ribosomal subunit of polyribosomes; may contribute to fragile X cognitive disability syndrome | dgacrrr | 7.01×10−3 |
| SRSF7 (serine/arginine-rich splicing factor 7) | critical for mRNA splicing as part of the spliceosome; involved in mRNA nuclear export and translation | acgacg | 6.44×10−3 |
| FMR1 (FMRP translational regulator 1) | associated with polyribosomes; involved in mRNA trafficking; negative regulator of translation | kgacarg | 7.53×10−3 |
| HNRNPM (heterogenous nuclear ribonucleoprotein M) | influences pre-mRNA processing, mRNA metabolism, and mRNA transport | gguugguu | 5.07×10−3 |
| YBX2 (Y-box binding protein 2) | regulates the stability and translation of germ cell mRNAs | aacawcd | 1.68×10−3 |
| RBM24 (RNA binding motif protein 24) | a tissue-specific splicing regulator; involved in mRNA stability | wgwgugd | 5.83×10−4 |
| PABPC4 (poly(A) binding protein cytoplasmic 4) | regulates stability of labile mRNA species in activated T cells; involved in translation in platelets and megakaryocytes | aaaaaar | 5.61×10−3 |
| HuR (human antigen R) | stabilizes mRNA by binding AU rich elements (AREs) | uukruuu | 4.61×10−3 |
| Protein | Description | Motif | P-value |
|---|---|---|---|
| ENOX1 (ecto-NOX disulfide-thiol exchanger 1) | involved in plasma membrane electron transport (PMET) pathways with alternating hydroquinone (NADH) oxidase and protein disulfide-thiol interchange activities | hrkacag | 5.17×10−4 |
| CNOT4 (CCR4-NOT transcription complex subunit 4) | subunit of CCR4-NOT complex; E3 ubiquitin ligase activity; interacts with CNOT1 | gacaga | 5.14×10−4 |
| SRSF3 (serine/arginine-rich splicing factor 3) | critical for mRNA splicing as part of the spliceosome; involved in mRNA nuclear export and translation | wcwwc | 4.00×10−4 |
| KHDRBS2 (KH RNA binding domain containing, signal transduction associated 2) | influences mRNA splice site selection and exon inclusion | rauaaam | 5.90×10−3 |
| HuR (human antigen R) | stabilizes mRNA by binding AREs | uukruuu | 7.12×10−3 |
| RBMS3 (RNA-binding motif, single-stranded-interacting protein 3) | (may be) involved in the control of RNA metabolism | hauaua | 1.89×10−3 |
| KHDRBS1 (KH RNA binding domain containing, signal transduction associated 1) | involved in alternative splicing, cell cycle regulation, RNA 3'-end formation, tumorigenesis, and regulation of human immunodeficiency virus (HIV) gene expression | auaaaav | 2.66×10−4 |
| PABPN1 (poly(A) binding protein nuclear 1) | binds to nascent poly(A) tails and directs polymerization of poly(A) tails at the 3' ends of eukaryotic transcripts | araaga | 9.11×10−3 |
| RBM42 (RNA binding motif protein 42) | involved in maintaining cellular ATP levels under stress by protecting target mRNAs | aacuamg | 4.44×10−4 |
miRNA[edit]
Several miRNAs have binding sites in the conserved regions of the 3' UTR of SLC46A3. The following miRNAs can negatively regulate the expression of the mRNA via RNA silencing.[66] Silencing mechanisms include mRNA cleavage and translation repression based on the level of complementarity between the miRNA and mRNA target sequences.
| Name | Binding Site Sequence | Target Score |
|---|---|---|
| hsa-miR-494-3p | ATGTTTCA | 97 |
| hsa-miR-106b-5p | GCACTTT – GCACTTT – GCACTTTA | 94 |
| hsa-miR-7159-5p | TTGTTGA – TTGTTGAA | 94 |
| hsa-miR-5680 | ATTTCTA – CATTTCT | 91 |
| hsa-miR-4477b | TCCTTAAA – TCCTTAAA | 91 |
| hsa-miR-660-5p | AATGGGT – AATGGGTA | 89 |
| hsa-miR-4319 | CTCAGGGA | 89 |
| hsa-miR-7162-3p | ACCTCAG | 89 |
| hsa-miR-137-3p | AGCAATAA | 88 |
| hsa-miR-6071 | CAGCAGAA | 88 |
| hsa-miR-597-3p | GAGAACCA | 86 |
| hsa-miR-510-3p | TTTCAAA – GTTTCAAA | 86 |
Secondary Structure[edit]

The secondary structure of RNA holds both structural and functional significance.[69] Among various secondary structure motifs, the stem-loop structure (hairpin loop) is often conserved across species due to its role in RNA folding, protecting structural stability, and providing recognition sites for RBPs.[70] The 5' UTR region of SLC46A3 has 7 stem-loop structures identified and 3' UTR region a total of 10.[71] The majority of the binding sites of RBPs and miRNAs given above are located at a stem-loop structure, which is also true for the poly(A) signal at the 3' end.
Protein Level Regulation[edit]
Subcellular Localization[edit]
The k-Nearest Neighbor (k-NN) prediction by PSORTII predicts SLC46A3 to be mainly located at the plasma membrane (78.3%) and ER (17.4%), but also possibly at the mitochondrion (4.3%).[72] Immunofluorescent staining of SLC46A3 shows positivity in the plasma membrane, cytoplasm, and actin filaments, although positivity in the latter two is most likely due to the process of the protein being transported by myosin from the ER to the plasma membrane; myosin transports cargo-containing membrane vesicles along actin filaments.[14][73]
Post-Translational Modification[edit]

The SLC46A3 protein contains a signal peptide that facilitates co-translational translocation and is cleaved between Thr20 and Gly21.[74][75] The resulting mature protein, 441 amino acids of length, is subject to further post-translational modifications (PTMs). The sequence has 3 N-glycosylation sites (Asn38, Asn46, Asn53), which are all located in the non-cytoplasmic region flanked by the signal peptide and the first transmembrane domain.[76] Ridigity of the N-terminal region close to the membrane is increased by O-GalNAc at Thr25.[77][78] O-GlcNAc at sites Ser227, Thr231, Ser445, and Ser459 are involved in the regulation of signaling pathways.[79][80] In fact, Ser445 and Ser459 are also subject to phosphorylation, where both sites are associated with casein kinase II (CKII), suggesting a crosstalking network that regulates protein activity.[81][82][83] Other highly conserved phosphorylation sites include Thr166, Ser233, Ser253, and Ser454, which are most likely targeted by kinases protein kinase C (PKC), CKII, PKC, and CKI/II, respectively. Conserved glycation sites at epsilon amino groups of lysines are predicted at Lys101, Lys239, and Lys374 with possible disrupting effects on molecular conformation and function of the protein.[84][85] S-palmitoylation, which help the protein bind more tightly to the membrane by contributing to protein hydrophobicity and membrane association, is predicted at Cys261 and Cys438.[86][87][88][89] S-palmitoylation can also modulate protein-protein interactions of SLC46A3 by changing the affinity of the protein for lipid rafts.
Homology and Evolution[edit]
Paralogs[edit]
SLC46A1: Also known as the proton-coupled folate transporter, SLC46A3 transports folate and antifolate substrates across cell membranes in a pH-dependent manner.[90]
SLC46A2: Aliases include thymic stromal cotransporter homolog, TSCOT, and Ly110. SLC46A2 is involved in symporter activity[91] and is a transporter of the immune second messenger 2'3'-cGAMP.[92]
| Paralog | Estimated Date of Divergence (MYA) | Accession Number | Sequence Length (aa) | Sequence Identity (%) | Sequence Similarity (%) |
|---|---|---|---|---|---|
| SLC46A1 | 724 | NP_542400.2 | 459 | 31 | 49 |
| SLC46A2 | 810 | NP_149040.3 | 475 | 27 | 44 |
Orthologs[edit]
SLC46A3 is a highly conserved protein with orthologs as distant as fungi.[21][93] Closely related orthologs have been found in mammals with sequence similarities above 75% while moderately related orthologs come from species of birds, reptiles, amphibians, and fish with sequence similarities of 50-70%. More distantly related orthologs have sequence similarities below 50% and are invertebrates, placozoa, and fungi. The MFS, MFS_1, and transmembrane domains mostly remain conserved throughout species. A selected list of orthologs obtained through NCBI BLAST is shown in the table below.
| Genus and Species | Common Name | Taxonomic Group | Date of Divergence (MYA) | Accession Number | Sequence Length (aa) | Sequence Identity (%) | Sequence Similarity (%) |
|---|---|---|---|---|---|---|---|
| Homo sapiens | Human | Mammalia | 0 | NP_861450.1 | 461 | 100 | 100 |
| Macaca mulatta | Rhesus Monkey | Mammalia | 29 | XP_014976295.2 | 460 | 95 | 96 |
| Mus musculus | House Mouse | Mammalia | 90 | NP_001343931.1 | 460 | 75 | 86 |
| Ornithorhynchus anatinus | Platypus | Mammalia | 177 | XP_028904425.1 | 462 | 68 | 81 |
| Gallus gallus | Chicken | Aves | 312 | NP_001025999.1 | 464 | 51 | 69 |
| Pseudonaja textilis | Eastern Brown Snake | Reptilia | 312 | XP_026564717.1 | 461 | 44 | 63 |
| Xenopus tropicalis | Tropical Clawed Frog | Amphibia | 352 | XP_002934077.1 | 473 | 42 | 62 |
| Danio rerio | Zebrafish | Actinopterygii | 435 | XP_021329877.1 | 463 | 42 | 62 |
| Rhincodon typus | Whale Shark | Chondrichthyes | 473 | XP_020383213.1 | 456 | 39 | 56 |
| Anneissia japonica | Feather Star | Crinoidea | 684 | XP_033118008.1 | 466 | 29 | 47 |
| Pecten maximus | Great Scallop | Bivalvia | 797 | XP_033735180.1 | 517 | 24 | 40 |
| Drosophila navojoa | Fruit Fly | Insecta | 797 | XP_030245348.1 | 595 | 19 | 34 |
| Nematostella vectensis | Starlet Sea Anemone | Anthozoa | 824 | XP_001640625.1 | 509 | 28 | 46 |
| Schmidtea mediterranea | Flatworm | Rhabditophora | 824 | AKN21695.1 | 483 | 23 | 38 |
| Trichoplax adhaerens | Trichoplax | Tricoplacia | 948 | XP_002114167.1 | 474 | 19 | 36 |
| Chytriomyces confervae | C. confervae | Chytridiomycetes | 1105 | TPX75507.1 | 498 | 23 | 40 |
| Tuber magnatum | White Truffle | Pezizomycetes | 1105 | PWW79074.1 | 557 | 21 | 34 |
| Cladophialophora bantiana | C. bantiana | Eurotiomycetes | 1105 | XP_016623985.1 | 587 | 21 | 32 |
| Exophiala mesophila | Black Yeast | Eurotiomycetes | 1105 | RVX69813.1 | 593 | 19 | 32 |
| Aspergillus terreus | Mold | Eurotiomycetes | 1105 | GES65939.1 | 604 | 19 | 31 |
Evolutionary History[edit]
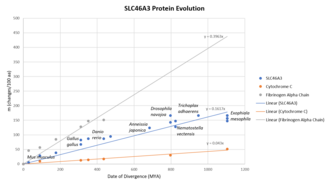
The SLC46A3 gene first appeared in fungi approximately 1105 million years ago (MYA).[21] It evolves at a relatively moderate speed. A 1% change in the protein sequence requires about 6.2 million years. The SLC46A3 gene evolves about 4 times faster than cytochrome c and 2.5 times slower than fibrinogen alpha chain.
Function[edit]
As an MFS protein, SLC46A3 is a membrane transporter, mainly involved in the movement of substrates across the lipid bilayer.[9] The protein works via secondary active transport, where the energy for transport is provided by an electrochemical gradient.[95]
A proposed function of SLC46A3 of rising importance is the direct transport of maytansine-based catabolites from the lysosome to the cytoplasm by binding the macrolide structure of maytansine.[96] Among the different types of antibody-drug conjugates (ADCs), maytansine-based noncleavable linker ADC catabolites, such as lysine-MCC-DM1, are particularly responsive to SLC46A3 activity.[17] The protein functions independent of the cell surface target or cell line, thus is most likely to recognize maytansine or a moiety within the maytansine scaffold. Through transmembrane transport activity, the protein regulates catabolite concentration in the lysosome. In addition, SLC46A3 expression has been identified as a mechanism for resistance to ADCs with noncleavable maytansinoid and pyrrolobenzodiazepine warheads.[97] Although subcellular localization predictions have failed to identify the lysosome as a final destination of the protein, the YXXphi motif identified in the protein sequence has shown to direct lysosomal sorting.[39]
SLC46A3 may be involved in plasma membrane electron transport (PMET), a plasma membrane analog of the mitochondrial electron transport chain (ETC) that oxidizes intracellular NADH and contributes to aerobic energy production by supporting glycolytic ATP production.[98] The 3' UTR region of SLC46A3 includes a binding site for ENOX1, a protein highly involved in PMET.[65][99] The C-(X)2-C motif in the protein sequence also suggests possible oxidoreductase activity.[36]
Interacting Proteins[edit]
SLC46A3 has been found to generally interact with proteins involved in membrane transport, immune response, catalytic activity, or oxidation of substrates.[100] Some of the most definite and clinically important interactions include the following proteins.
- CD79A: An interaction with CD79A was identified in a yeast-two hybrid (Y2H) screen with a confidence score of 0.632 by the human binary protein interactome (HuRI).[101] Also known as B-cell antigen receptor complex-associated protein alpha chain, CD79A, together with CD79B, forms the B-cell antigen receptor (BCR) by covalently associating with surface immunoglobulin (Ig).[102] The BCR responds to antigens and initiates signal transduction cascades.[103]
- LGALS3: High-throughput affinity purification-mass spectrometry (AP-MS) identified an interaction between SLC46A3 and LGALS3 with an interaction score of 0.761, classified as high-confidence interacting proteins (HCIPs) by CompPASS-Plus.[104] Also known as galectin-3 (Gal3), LGALS3 participates in various cellular functions including apoptosis, innate immunity, cell adhesion, and T-cell regulation.[105] The protein is involved in antimicrobial activity against bacteria and fungi and has been identified as a negative regulator of mast cell degranulation. LGALS3 is highly upregulated in glioblastoma tissue and brains of Altzheimer's disease patients.
- NSP2: A high-throughput Y2H screening of the SARS-CoV ORFeome and host proteins isolated a single-hit interaction between NSP2 and SLC46A3 with a LUMIER z-score of -0.5.[16] Short for non-structural protein 2, NSP2 is one of the many non-structural proteins encoded in the orf1ab polyprotein.[106][107] NSP2 alters the host cell environment rather than contribute directly to viral replication. The protein interacts with prohibitin 1 (PHB1) and PHB2.
Variants[edit]
SNPs are a very common type of genetic variation and are silent most of the time.[108] However, certain SNPs in the conserved or functionally important regions of the gene may have adverse effects on gene expression and function. Some of the SNPs with potentially damaging effects identified in the coding sequence of SLC46A3 are shown in the table below.
| SNP | mRNA position | Amino Acid Position | Base Change | Amino Acid Change | Function | Description |
|---|---|---|---|---|---|---|
| rs1456067444 | 554 | 1 | [T/G] | [M/R] | missense | start codon |
| rs749501877 | 679 | 46 | [A/G] | [N/S] | missense | N-glycosylation site |
| rs776889950 | 897 | 119 | [T/G] | [C/G] | missense | C-(X)2-C motif |
| rs1403613207 | 967 | 142 | [G/A] | [G/D] | missense | conserved substrate translocation pore |
| rs764198426 | 1322 | 261 | [CT/-] | [C/F] | frameshift | S-palmitoylation site |
| rs1373735793 | 1878 | 446 | [T/C] | [Y/H] | missense | YXXphi motif & STAP1 SH2 domain binding motif |
| rs1342327615 | 1906 | 455 | [G/A] | [S/N] | missense | phosphorylation & O-GlcNAc site |
| rs757225275 | 1917 | 459 | [T/G]
[T/-] |
[S/A]
[S/Q] |
missense
frameshift |
phosphorylation & O-GlcNAc site |
f*The coordinates/positions are for GRCh38.p7.
Clinical Significance[edit]
Cancer/Tumor[edit]
The clinical significance of SLC46A3 surrounds the protein's activity as a transporter of maytansine-based ADC catabolites.[96] shRNA screens employing two libraries identified SLC46A3 as the only hit as a mediator of noncleavable maytansine-based ADC-dependent cytotoxicity, with q-values of 1.18×10−9 and 9.01×10−3.[17] Studies show either lost or significantly reduced SLC46A3 expression (-2.79 fold decrease by microarray with p-value 5.80×10−8) in T-DM1 (DM1 payload attached to antibody trastuzumab)-resistant breast cancer cells (KPL-4 TR).[11] In addition, siRNA knockdown in human breast tumor cell line BT-474M1 also results in resistance to T-DM1. Such association between loss of SLC46A3 expression and resistance to ADCs also applies to pyrrolobenzodiazepine warheads, signifying the important role of SLC46A3 in cancer treatment.[97]
CDP, one of SLC46A3's transcription factors, works as a tumor suppressor where CDP deficiency activates phosphoinositide 3-kinase (PI3K) signaling that leads to tumor growth.[110] The loss of heterozygosity and mutations of CDP are also associated with a variety of cancers.[111]
Prostate Cancer[edit]
Microarray analysis of SLC46A3 in two different prostate cancer cell lines, LNCaP (androgen-dependent) and DU145 (androgen-independent), show SLC46A3 expression in DU145 to be about 5 times as high as in LNCaP for percentile ranks and 1.5 times as high for transformed counts, demonstrating an association between SLC46A3 and accelerated cell growth of prostate cancer cells.[12] SLC46A3 possibly contributes to the androgen-independent manner of cancer development.
Hepatocellular Carcinoma (HCC)[edit]
SLC46A3 was found to be down-regulated in 83.2% of human HCC tissues based on western blot scores and qRT-PCR results on mRNA expression (p < 0.0001).[13] Overexpression of the gene also reduced resistance to sorafenib treatment and improved overall survival rate (p = 0.00085).
Papilloma & Glioma[edit]
Western blot analysis supports substantially strong expression of SLC46A3 in papilloma and glioma cells when compared to expression in the liver, one of the organs where the gene is most highly expressed.[14]
Obesity[edit]
A genome-wide association study on obesity identified 10 variants in the flanking 5′UTR region of SLC46A3 that were highly associated with diet fat (% energy) (p = 1.36×10−6 - 9.57×10−6).[15] In diet-induced obese (DIO) mice, SLC46A3 shows decreased gene expression following c-Jun N-terminal kinase 1 (JNK1) depletion, suggesting possible roles in insulin resistance as well as glucose/triglyceride homeostasis.[112]
SARS-CoV & SARS-CoV-2[edit]
Understanding the interaction between SLC46A3 and NSP2 in addition to the functions of each protein is critical to gaining insight into the pathogenesis of coronaviruses, namely SARS-CoV and SARS-CoV-2. The NSP2 protein domain resides in a region of the coronavirus replicase that is not particularly conserved across coronaviruses, and thus the altering protein sequence leads to significant changes in protein structure, leading to structural and functional variability.[106]
See also[edit]
References[edit]
- ^ a b c GRCh38: Ensembl release 89: ENSG00000139508 – Ensembl, May 2017
- ^ a b c GRCm38: Ensembl release 89: ENSMUSG00000029650 – Ensembl, May 2017
- ^ "Human PubMed Reference:". National Center for Biotechnology Information, U.S. National Library of Medicine.
- ^ "Mouse PubMed Reference:". National Center for Biotechnology Information, U.S. National Library of Medicine.
- ^ a b c d e "SLC46A3". NCBI (National Center for Biotechnology Information) Gene.
- ^ a b "SLC46A3 Gene". GeneCards The Human Gene Database.
- ^ Nakai K, Horton P (2007). "Computational Prediction of Subcellular Localization". Protein Targeting Protocols. Methods in Molecular Biology. Vol. 390. Totowa, NJ: Humana Press. pp. 429–466. doi:10.1007/1-59745-466-4_29. ISBN 978-1-58829-702-0.
- ^ a b c "solute carrier family 46 member 3 isoform a precursor [Homo sapiens]". NCBI (National Center for Biotechnology Information) Protein.
- ^ a b "SLC46A3". OMIM (Online Mendelian Inheritance in Man).
- ^ a b "MFS". NCBI (National Center for Biotechnology Information) CDD (Conserved Domain Database).
- ^ a b Li G, Guo J, Shen BQ, Yadav DB, Sliwkowski MX, Crocker LM, et al. (July 2018). "Mechanisms of Acquired Resistance to Trastuzumab Emtansine in Breast Cancer Cells". Molecular Cancer Therapeutics. 17 (7): 1441–1453. doi:10.1158/1535-7163.mct-17-0296. PMID 29695635.
- ^ a b Kanaoka R, Kushiyama A, Seno Y, Nakatsu Y, Matsunaga Y, Fukushima T, et al. (2015-06-03). "Pin1 Inhibitor Juglone Exerts Anti-Oncogenic Effects on LNCaP and DU145 Cells despite the Patterns of Gene Regulation by Pin1 Differing between These Cell Lines". PLOS ONE. 10 (6): e0127467. Bibcode:2015PLoSO..1027467K. doi:10.1371/journal.pone.0127467. PMC 4454534. PMID 26039047.
- ^ a b Zhao Q, Zheng B, Meng S, Xu Y, Guo J, Chen LJ, et al. (June 2019). "Increased expression of SLC46A3 to oppose the progression of hepatocellular carcinoma and its effect on sorafenib therapy". Biomedicine & Pharmacotherapy. 114: 108864. doi:10.1016/j.biopha.2019.108864. PMID 30981107.
- ^ a b c d "SLC46A3 Polyclonal Antibody". ThermoFisher Scientific.
- ^ a b Comuzzie AG, Cole SA, Laston SL, Voruganti VS, Haack K, Gibbs RA, Butte NF (2012-12-14). "Novel genetic loci identified for the pathophysiology of childhood obesity in the Hispanic population". PLOS ONE. 7 (12): e51954. Bibcode:2012PLoSO...751954C. doi:10.1371/journal.pone.0051954. PMC 3522587. PMID 23251661.
- ^ a b Pfefferle S, Schöpf J, Kögl M, Friedel CC, Müller MA, Carbajo-Lozoya J, et al. (October 2011). "The SARS-coronavirus-host interactome: identification of cyclophilins as target for pan-coronavirus inhibitors". PLOS Pathogens. 7 (10): e1002331. doi:10.1371/journal.ppat.1002331. PMC 3203193. PMID 22046132.
- ^ a b c Hamblett KJ, Jacob AP, Gurgel JL, Tometsko ME, Rock BM, Patel SK, et al. (December 2015). "SLC46A3 Is Required to Transport Catabolites of Noncleavable Antibody Maytansine Conjugates from the Lysosome to the Cytoplasm". Cancer Research. 75 (24): 5329–40. doi:10.1158/0008-5472.can-15-1610. PMID 26631267.
- ^ a b c Fagerberg L, Hallström BM, Oksvold P, Kampf C, Djureinovic D, Odeberg J, et al. (February 2014). "Analysis of the human tissue-specific expression by genome-wide integration of transcriptomics and antibody-based proteomics". Molecular & Cellular Proteomics. 13 (2): 397–406. doi:10.1074/mcp.m113.035600. PMC 3916642. PMID 24309898.
- ^ a b c Duff MO, Olson S, Wei X, Garrett SC, Osman A, Bolisetty M, Plocik A, Celniker SE, Graveley BR (May 2015). "Genome-wide identification of zero nucleotide recursive splicing in Drosophila". Nature. 521 (7552): 376–9. Bibcode:2015Natur.521..376D. doi:10.1038/nature14475. PMC 4529404. PMID 25970244.
- ^ "SLC46A3". AceView.
- ^ a b c d e "BLAST: Basic Local Alignment Search Tool". NCBI (National Center for Biotechnology Information).
- ^ "Variation Viewer (GRCh38)". NCBI (National Center for Biotechnology Information).
- ^ "SLC46A3". PAXdb.
- ^ a b c "SLC46A3". Genomatix: ElDorado. Archived from the original on 2021-08-26. Retrieved 2020-08-01.
- ^ Pruitt K, Brown G, Tatusova T, Maglott D (2012-04-06). The Reference Sequence (RefSeq) Database. National Center for Biotechnology Information (US).
- ^ a b "Homo sapiens solute carrier family 46 member 3 (SLC46A3), transcript variant 1, mRNA". NCBI (National Center for Biotechnology Information) Nucleotide. 14 December 2020.
- ^ "Homo sapiens solute carrier family 46 member 3 (SLC46A3), transcript variant 2, mRNA". NCBI (National Center for Biotechnology Information) Nucleotide. 13 December 2020.
- ^ "Homo sapiens solute carrier family 46 member 3 (SLC46A3), transcript variant 3, mRNA". NCBI (National Center for Biotechnology Information) Nucleotide. 2 July 2020.
- ^ "PREDICTED: Homo sapiens solute carrier family 46 member 3 (SLC46A3), transcript variant X1, mRNA". NCBI (National Center for Biotechnology Information) Nucleotide. 16 May 2021.
- ^ a b c "solute carrier family 46 member 3 isoform a precursor [Homo sapiens]". NCBI (National Center for Biotechnology Information) Protein.
- ^ a b "solute carrier family 46 member 3 isoform b precursor [Homo sapiens]". NCBI (National Center for Biotechnology Information) Protein.
- ^ a b "solute carrier family 46 member 3 isoform X1 [Homo sapiens]". NCBI (National Center for Biotechnology Information) Protein.
- ^ a b c d e Brendel V, Bucher P, Nourbakhsh IR, Blaisdell BE, Karlin S (March 1992). "Methods and algorithms for statistical analysis of protein sequences". Proceedings of the National Academy of Sciences of the United States of America. 89 (6): 2002–6. Bibcode:1992PNAS...89.2002B. doi:10.1073/pnas.89.6.2002. PMC 48584. PMID 1549558.
- ^ Gasteiger E, Hoogland C, Gattiker A, Duvaud S, Wilkins MR, Appel RD, Bairoch A (2005), "Protein Identification and Analysis Tools on the ExPASy Server", The Proteomics Protocols Handbook, Totowa, NJ: Humana Press, pp. 571–607, doi:10.1385/1-59259-890-0:571, ISBN 978-1-58829-343-5
- ^ Alberts B, Johnson A, Lewis J, Raff M, Roberts K, Walter P (2002). "Membrane Proteins". Molecular Biology of the Cell (4th ed.). Garland Science.
- ^ a b Miseta A, Csutora P (August 2000). "Relationship between the occurrence of cysteine in proteins and the complexity of organisms". Molecular Biology and Evolution. 17 (8): 1232–9. doi:10.1093/oxfordjournals.molbev.a026406. PMID 10908643.
- ^ a b Kumar M, Gouw M, Michael S, Sámano-Sánchez H, Pancsa R, Glavina J, et al. (January 2020). "ELM-the eukaryotic linear motif resource in 2020". Nucleic Acids Research. 48 (D1): D296–D306. doi:10.1093/nar/gkz1030. PMC 7145657. PMID 31680160.
- ^ "TRG_ENDOCYTIC_2". ELM (The Eukaryotic Linear Motif resource for Functional Sites in Proteins).
- ^ a b Pandey KN (October 2010). "Small peptide recognition sequence for intracellular sorting". Current Opinion in Biotechnology. 21 (5): 611–20. doi:10.1016/j.copbio.2010.08.007. PMC 2997389. PMID 20817434.
- ^ "LIG_SH2_STAP1". ELM (The Eukaryotic Linear Motif resource for Functional Sites in Proteins).
- ^ Eisenberg D, Weiss RM, Terwilliger TC (January 1984). "The hydrophobic moment detects periodicity in protein hydrophobicity". Proceedings of the National Academy of Sciences of the United States of America. 81 (1): 140–4. Bibcode:1984PNAS...81..140E. doi:10.1073/pnas.81.1.140. PMC 344626. PMID 6582470.
- ^ Zimmermann L, Stephens A, Nam SZ, Rau D, Kübler J, Lozajic M, et al. (July 2018). "A Completely Reimplemented MPI Bioinformatics Toolkit with a New HHpred Server at its Core". Journal of Molecular Biology. 430 (15): 2237–2243. doi:10.1016/j.jmb.2017.12.007. PMID 29258817. S2CID 22415932.
- ^ Reithmeier RA (1996). "Assembly of proteins into membranes". Biochemistry of Lipids, Lipoproteins and Membranes. New Comprehensive Biochemistry. Vol. 31. Elsevier. pp. 425–471. doi:10.1016/s0167-7306(08)60523-2. ISBN 978-0-444-82359-5.
- ^ Biggin PC, Sansom MS (February 1999). "Interactions of alpha-helices with lipid bilayers: a review of simulation studies". Biophysical Chemistry. 76 (3): 161–83. doi:10.1016/s0301-4622(98)00233-6. PMID 10074693.
- ^ Omasits U, Ahrens CH, Müller S, Wollscheid B (March 2014). "Protter: interactive protein feature visualization and integration with experimental proteomic data". Bioinformatics. 30 (6): 884–6. doi:10.1093/bioinformatics/btt607. hdl:20.500.11850/82692. PMID 24162465.
- ^ "Q7Z3Q1 (S46A3_HUMAN)". UniProt.
- ^ Yang J, Zhang Y (July 2015). "I-TASSER server: new development for protein structure and function predictions". Nucleic Acids Research. 43 (W1): W174-81. doi:10.1093/nar/gkv342. PMC 4489253. PMID 25883148.
- ^ Zhang Y, Skolnick J (2005-04-11). "TM-align: a protein structure alignment algorithm based on the TM-score". Nucleic Acids Research. 33 (7): 2302–9. doi:10.1093/nar/gki524. PMC 1084323. PMID 15849316.
- ^ a b "I-TASSER results". Zhang Lab.
- ^ Zhang C, Freddolino PL, Zhang Y (July 2017). "COFACTOR: improved protein function prediction by combining structure, sequence and protein-protein interaction information". Nucleic Acids Research. 45 (W1): W291–W299. doi:10.1093/nar/gkx366. PMC 5793808. PMID 28472402.
- ^ Yang J, Roy A, Zhang Y (October 2013). "Protein-ligand binding site recognition using complementary binding-specific substructure comparison and sequence profile alignment". Bioinformatics. 29 (20): 2588–95. doi:10.1093/bioinformatics/btt447. PMC 3789548. PMID 23975762.
- ^ Latchman DS (2004). "Methods for Studying Transcription Factors". Eukaryotic Transcription Factors. Vol. 270. Elsevier. pp. 23–53. doi:10.1016/b978-012437178-1/50008-4. ISBN 978-0-12-437178-1. PMC 1131717. PMID 2119171.
{{cite book}}:|journal=ignored (help) - ^ a b c "SLC46A3 Transcription Factor Binding Sites". Genomatix: MatInspector.
- ^ Miller DM, Thomas SD, Islam A, Muench D, Sedoris K (October 2012). "c-Myc and cancer metabolism". Clinical Cancer Research. 18 (20): 5546–53. doi:10.1158/1078-0432.CCR-12-0977. PMC 3505847. PMID 23071356.
- ^ Ellis T, Gambardella L, Horcher M, Tschanz S, Capol J, Bertram P, et al. (September 2001). "The transcriptional repressor CDP (Cutl1) is essential for epithelial cell differentiation of the lung and the hair follicle". Genes & Development. 15 (17): 2307–19. doi:10.1101/gad.200101. PMC 312776. PMID 11544187.
- ^ Wang GZ, Zhang W, Fang ZT, Zhang W, Yang MJ, Yang GW, et al. (July 2014). "Arsenic trioxide: marked suppression of tumor metastasis potential by inhibiting the transcription factor Twist in vivo and in vitro". Journal of Cancer Research and Clinical Oncology. 140 (7): 1125–36. doi:10.1007/s00432-014-1659-6. PMID 24756364. S2CID 6332740.
- ^ "Illumina bodyMap2 transcriptome". NCBI (National Center for Biotechnology Information) BioProject.
- ^ Szabo L, Morey R, Palpant NJ, Wang PL, Afari N, Jiang C, et al. (December 2016). "Erratum to: Statistically based splicing detection reveals neural enrichment and tissue-specific induction of circular RNA during human fetal development". Genome Biology. 17 (1): 263. doi:10.1186/s13059-016-1123-9. PMC 5165717. PMID 27993159.
- ^ Su AI, Wiltshire T, Batalov S, Lapp H, Ching KA, Block D, et al. (April 2004). "A gene atlas of the mouse and human protein-encoding transcriptomes". Proceedings of the National Academy of Sciences of the United States of America. 101 (16): 6062–7. Bibcode:2004PNAS..101.6062S. doi:10.1073/pnas.0400782101. PMC 395923. PMID 15075390.
- ^ "SLC46A3". GenePaint.
- ^ "SLC46A3 (Mouse Brain)". Allen Brain Atlas.
- ^ "Slc46a3 ISH: Mus musculus, Male, P4, variable". Allen Brain Atlas.
- ^ "Slc46a3 ISH: Mus musculus, Male, P56, variable". Allen Brain Atlas.
- ^ Brinegar AE, Cooper TA (September 2016). "Roles for RNA-binding proteins in development and disease". Brain Research. 1647: 1–8. doi:10.1016/j.brainres.2016.02.050. PMC 5003702. PMID 26972534.
- ^ a b c Paz I, Kosti I, Ares M, Cline M, Mandel-Gutfreund Y (July 2014). "RBPmap: a web server for mapping binding sites of RNA-binding proteins". Nucleic Acids Research. 42 (Web Server issue): W361-7. doi:10.1093/nar/gku406. PMC 4086114. PMID 24829458.
- ^ Macfarlane LA, Murphy PR (November 2010). "MicroRNA: Biogenesis, Function and Role in Cancer". Current Genomics. 11 (7): 537–61. doi:10.2174/138920210793175895. PMC 3048316. PMID 21532838.
- ^ Chen Y, Wang X (January 2020). "miRDB: an online database for prediction of functional microRNA targets". Nucleic Acids Research. 48 (D1): D127–D131. doi:10.1093/nar/gkz757. PMC 6943051. PMID 31504780.
- ^ "SLC46A3". miRDB.
- ^ Vandivier LE, Anderson SJ, Foley SW, Gregory BD (April 2016). "The Conservation and Function of RNA Secondary Structure in Plants". Annual Review of Plant Biology. 67 (1): 463–88. doi:10.1146/annurev-arplant-043015-111754. PMC 5125251. PMID 26865341.
- ^ Control of Messenger RNA Stability. 1993. doi:10.1016/c2009-0-03269-3. ISBN 9780120847822.
- ^ Zuker M (July 2003). "Mfold web server for nucleic acid folding and hybridization prediction". Nucleic Acids Research. 31 (13): 3406–15. doi:10.1093/nar/gkg595. PMC 169194. PMID 12824337.
- ^ Nakai K, Horton P (2007). "Computational Prediction of Subcellular Localization". Protein Targeting Protocols. Methods in Molecular Biology. Vol. 390. Totowa, NJ: Humana Press. pp. 429–466. doi:10.1007/1-59745-466-4_29. ISBN 978-1-58829-702-0.
- ^ "The Cell: A Molecular Approach. Sixth Edition. By Geoffrey M. Cooper and Robert E. Hausman. Sunderland (Massachusetts): Sinauer Associates. $142.95. xxv + 832 p.; ill.; index. [A Companion Website is available.] 2013". The Quarterly Review of Biology. 89 (4): 399. 2014. doi:10.1086/678645. ISBN 978-0-87893-964-0. ISSN 0033-5770.
- ^ Almagro Armenteros JJ, Tsirigos KD, Sønderby CK, Petersen TN, Winther O, Brunak S, et al. (April 2019). "SignalP 5.0 improves signal peptide predictions using deep neural networks" (PDF). Nature Biotechnology. 37 (4): 420–423. doi:10.1038/s41587-019-0036-z. PMID 30778233. S2CID 216678118.
- ^ Käll L, Krogh A, Sonnhammer EL (May 2004). "A combined transmembrane topology and signal peptide prediction method". Journal of Molecular Biology. 338 (5): 1027–36. doi:10.1016/j.jmb.2004.03.016. PMID 15111065.
- ^ Julenius K, Johansen MB, Zhang Y, Brunak S, Gupta R (2009). "Prediction of Glycosylation Sites in Proteins". Bioinformatics for Glycobiology and Glycomics. Chichester, UK: John Wiley & Sons, Ltd. pp. 163–192. doi:10.1002/9780470029619.ch9. ISBN 978-0-470-02961-9.
- ^ Steentoft C, Vakhrushev SY, Joshi HJ, Kong Y, Vester-Christensen MB, Schjoldager KT, et al. (May 2013). "Precision mapping of the human O-GalNAc glycoproteome through SimpleCell technology". The EMBO Journal. 32 (10): 1478–88. doi:10.1038/emboj.2013.79. PMC 3655468. PMID 23584533.
- ^ Essentials of glycobiology. Varki, Ajit (Third ed.). Cold Spring Harbor, New York. 2017. ISBN 978-1-62182-132-8. OCLC 960166742.
{{cite book}}: CS1 maint: location missing publisher (link) CS1 maint: others (link) - ^ Gupta R, Brunak S (2001). "Prediction of glycosylation across the human proteome and the correlation to protein function". Pacific Symposium on Biocomputing. Pacific Symposium on Biocomputing. WORLD SCIENTIFIC: 310–22. doi:10.1142/9789812799623_0029. ISBN 978-981-02-4777-5. PMID 11928486.
- ^ Fisi V, Miseta A, Nagy T (2017). "The Role of Stress-Induced O-GlcNAc Protein Modification in the Regulation of Membrane Transport". Oxidative Medicine and Cellular Longevity. 2017: 1308692. doi:10.1155/2017/1308692. PMC 5804373. PMID 29456783.
- ^ Wang C, Xu H, Lin S, Deng W, Zhou J, Zhang Y, et al. (February 2020). "GPS 5.0: An Update on the Prediction of Kinase-specific Phosphorylation Sites in Proteins". Genomics, Proteomics & Bioinformatics. 18 (1): 72–80. doi:10.1016/j.gpb.2020.01.001. PMC 7393560. PMID 32200042.
- ^ Blom N, Gammeltoft S, Brunak S (December 1999). "Sequence and structure-based prediction of eukaryotic protein phosphorylation sites". Journal of Molecular Biology. 294 (5): 1351–62. doi:10.1006/jmbi.1999.3310. PMID 10600390.
- ^ Blom N, Sicheritz-Pontén T, Gupta R, Gammeltoft S, Brunak S (June 2004). "Prediction of post-translational glycosylation and phosphorylation of proteins from the amino acid sequence". Proteomics. 4 (6): 1633–49. doi:10.1002/pmic.200300771. PMID 15174133. S2CID 18810164.
- ^ Johansen MB, Kiemer L, Brunak S (September 2006). "Analysis and prediction of mammalian protein glycation". Glycobiology. 16 (9): 844–53. doi:10.1093/glycob/cwl009. PMID 16762979.
- ^ Chen JH, Lin X, Bu C, Zhang X (2018-10-10). "Role of advanced glycation end products in mobility and considerations in possible dietary and nutritional intervention strategies". Nutrition & Metabolism. 15 (1): 72. doi:10.1186/s12986-018-0306-7. PMC 6180645. PMID 30337945.
- ^ Xie Y, Zheng Y, Li H, Luo X, He Z, Cao S, et al. (June 2016). "GPS-Lipid: a robust tool for the prediction of multiple lipid modification sites". Scientific Reports. 6 (1): 28249. Bibcode:2016NatSR...628249X. doi:10.1038/srep28249. PMC 4910163. PMID 27306108.
- ^ Aicart-Ramos C, Valero RA, Rodriguez-Crespo I (December 2011). "Protein palmitoylation and subcellular trafficking". Biochimica et Biophysica Acta (BBA) - Biomembranes. 1808 (12): 2981–94. doi:10.1016/j.bbamem.2011.07.009. PMID 21819967.
- ^ Ren J, Wen L, Gao X, Jin C, Xue Y, Yao X (November 2008). "CSS-Palm 2.0: an updated software for palmitoylation sites prediction". Protein Engineering, Design & Selection. 21 (11): 639–44. doi:10.1093/protein/gzn039. PMC 2569006. PMID 18753194.
- ^ Guan X, Fierke CA (December 2011). "Understanding Protein Palmitoylation: Biological Significance and Enzymology". Science China Chemistry. 54 (12): 1888–1897. doi:10.1007/s11426-011-4428-2. PMC 4240533. PMID 25419213.
- ^ "SLC46A1". NCBI (National Center for Biotechnology Information) Gene.
- ^ "SLC46A2". NCIB (National Center for Biotechnology Information) Gene.
- ^ Cordova, Anthony F.; Ritchie, Christopher; Böhnert, Volker; Li, Lingyin (June 23, 2021). "Human SLC46A2 Is the Dominant cGAMP Importer in Extracellular cGAMP-Sensing Macrophages and Monocytes". ACS Cent Sci. 7 (6): 1073–1088. doi:10.1021/acscentsci.1c00440. PMC 8228594. PMID 34235268.
- ^ a b c Needleman SB, Wunsch CD (March 1970). "A general method applicable to the search for similarities in the amino acid sequence of two proteins". Journal of Molecular Biology. 48 (3): 443–53. doi:10.1016/0022-2836(70)90057-4. PMID 5420325.
- ^ Kumar S, Stecher G, Suleski M, Hedges SB (July 2017). "TimeTree: A Resource for Timelines, Timetrees, and Divergence Times". Molecular Biology and Evolution. 34 (7): 1812–1819. doi:10.1093/molbev/msx116. PMID 28387841.
- ^ Pao SS, Paulsen IT, Saier MH (March 1998). "Major facilitator superfamily". Microbiology and Molecular Biology Reviews. 62 (1): 1–34. doi:10.1128/mmbr.62.1.1-34.1998. PMC 98904. PMID 9529885.
- ^ a b Bissa B, Beedle AM, Govindarajan R (November 2016). "Lysosomal solute carrier transporters gain momentum in research". Clinical Pharmacology and Therapeutics. 100 (5): 431–436. doi:10.1002/cpt.450. PMC 5056150. PMID 27530302.
- ^ a b Kinneer K, Meekin J, Tiberghien AC, Tai YT, Phipps S, Kiefer CM, et al. (December 2018). "SLC46A3 as a Potential Predictive Biomarker for Antibody-Drug Conjugates Bearing Noncleavable Linked Maytansinoid and Pyrrolobenzodiazepine Warheads". Clinical Cancer Research. 24 (24): 6570–6582. doi:10.1158/1078-0432.ccr-18-1300. PMID 30131388.
- ^ Herst PM, Berridge MV (December 2006). "Plasma membrane electron transport: a new target for cancer drug development". Current Molecular Medicine. 6 (8): 895–904. doi:10.2174/156652406779010777. PMID 17168740. Retrieved 2020-08-01.
- ^ "ENOX1 ecto-NOX disulfide-thiol exchanger 1 [ Homo sapiens (human) ]". NCBI (National Center for Biotechnology Information) Gene.
- ^ "Figure S6: Predicted secondary structure of CoV-RMEN using CFSSP:Chou and Fasman secondary structure prediction server". doi:10.7717/peerj.9572/supp-13.
{{cite journal}}: Cite journal requires|journal=(help) - ^ Luck K, Kim DK, Lambourne L, Spirohn K, Begg BE, Bian W, et al. (April 2020). "A reference map of the human binary protein interactome". Nature. 580 (7803): 402–408. Bibcode:2020Natur.580..402L. doi:10.1038/s41586-020-2188-x. PMC 7169983. PMID 32296183.
- ^ "CD79A CD79a molecule [ Homo sapiens (human) ]". NCBI (National Center for Biotechnology Information) Gene.
- ^ "P11912 (CD79A_HUMAN)". UniProt.
- ^ Huttlin EL, Ting L, Bruckner RJ, Gebreab F, Gygi MP, Szpyt J, et al. (July 2015). "The BioPlex Network: A Systematic Exploration of the Human Interactome". Cell. 162 (2): 425–440. doi:10.1016/j.cell.2015.06.043. PMC 4617211. PMID 26186194.
- ^ "LGALS3 galectin 3 [ Homo sapiens (human) ]". NCBI (National Center for Biotechnology Information) Gene.
- ^ a b Graham RL, Sims AC, Baric RS, Denison MR (2006). "The NSP2 Proteins of Mouse Hepatitis Virus and Sars Coronavirus are Dispensable for Viral Replication". The Nidoviruses. Advances in Experimental Medicine and Biology. Vol. 581. Boston, MA: Springer US. pp. 67–72. doi:10.1007/978-0-387-33012-9_10. ISBN 978-0-387-26202-4. PMC 7123188. PMID 17037506.
- ^ "Review for "Therapeutic uncertainties in people with cardiometabolic diseases and severe acute respiratory syndrome coronavirus 2 ( <scp>SARS-CoV</scp> -2 or <scp>COVID</scp> -19)"". 2020-04-07. doi:10.1111/dom.14062/v1/review3. S2CID 219115750.
{{cite journal}}: Cite journal requires|journal=(help) - ^ Shen LX, Basilion JP, Stanton VP (July 1999). "Single-nucleotide polymorphisms can cause different structural folds of mRNA". Proceedings of the National Academy of Sciences of the United States of America. 96 (14): 7871–6. Bibcode:1999PNAS...96.7871S. doi:10.1073/pnas.96.14.7871. PMC 22154. PMID 10393914.
- ^ "SNP linked to Gene (geneID:283537) Via Contig Annotation". NCBI (National Center for Biotechnology Information) dbSNP Short Genetic Variations.
- ^ Wong CC, Martincorena I, Rust AG, Rashid M, Alifrangis C, Alexandrov LB, et al. (January 2014). "Inactivating CUX1 mutations promote tumorigenesis". Nature Genetics. 46 (1): 33–8. doi:10.1038/ng.2846. PMC 3874239. PMID 24316979.
- ^ Liu N, Sun Q, Wan L, Wang X, Feng Y, Luo J, Wu H (2020-05-29). "CUX1, A Controversial Player in Tumor Development". Frontiers in Oncology. 10: 738. doi:10.3389/fonc.2020.00738. PMC 7272708. PMID 32547943.
- ^ Yang R, Wilcox DM, Haasch DL, Jung PM, Nguyen PT, Voorbach MJ, et al. (August 2007). "Liver-specific knockdown of JNK1 up-regulates proliferator-activated receptor gamma coactivator 1 beta and increases plasma triglyceride despite reduced glucose and insulin levels in diet-induced obese mice". The Journal of Biological Chemistry. 282 (31): 22765–74. doi:10.1074/jbc.m700790200. PMID 17550900.
Further reading[edit]
- Chalasani N, Guo X, Loomba R, Goodarzi MO, Haritunians T, Kwon S, et al. (November 2010). "Genome-wide association study identifies variants associated with histologic features of nonalcoholic Fatty liver disease". Gastroenterology. 139 (5): 1567–76, 1576.e1-6. doi:10.1053/j.gastro.2010.07.057. PMC 2967576. PMID 20708005.
- Ma Y, Qi X, Du J, Song S, Feng D, Qi J, et al. (March 2009). "Identification of candidate genes for human pituitary development by EST analysis". BMC Genomics. 10: 109. doi:10.1186/1471-2164-10-109. PMC 2664823. PMID 19284880.



