
| |
| |
| Names | |
|---|---|
| IUPAC name
potassium tetranitrosyl-di-μ-sulfidodiiron(Fe–Fe)(2–)
| |
| Other names
Ferrate(2-), tetranitrosyldi-mu-thioxodi-, (Fe-Fe), dipotassium
| |
| Identifiers | |
3D model (JSmol)
|
|
| ChemSpider | |
PubChem CID
|
|
CompTox Dashboard (EPA)
|
|
| |
| |
| Properties | |
| Fe2N4K2O4S2 | |
| Molar mass | 374.04 g/mol |
| Appearance | Dark red crystals |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa).
| |
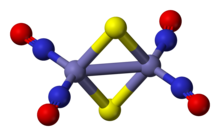
Roussin's red salt is the inorganic compound with the formula K2[Fe2S2(NO)4]. This metal nitrosyl was first described by Zacharie Roussin in 1858, making it one of the first synthetic iron-sulfur clusters.[1][2]
Structure and bonding[edit]
Roussin's red salt anion is an edge-shared bitetrahedron, wherein a pair Fe(NO)2 units are bridged by a pair of sulfide ligands. The Fe-NO bonds are linear indicating NO is acting as a three electron donor.[3] The diamagnetic compound obeys the 18-electron rule. The dark red colour of the complex is attributed to a number of charge-transfer interactions between the iron core and nitrosyl ligands.[4]
Synthesis[edit]
The French chemist Z. Roussin[5] first prepared this salt while investigating reactions between nitroprusside ion ([Fe(CN)5NO]2−) and sulfur.[6] The salt can be prepared by the reaction of sulfide salts with iron nitrosyl halides:[7]
- Fe2I2(NO)4 + 2Li2S → Li2Fe2S2(NO)4 + 2LiI
To obtain the "esters", the salt is alkylated:
- Li2Fe2S2(NO)4 + 2 RX → Fe2(SR)2(NO)4 + 2 LiX
Esters can also be easily be prepared from the reaction of Fe2I2(NO)4 with the thiol.
Occurrence and potential applications[edit]
It is found in nature as its "esters" with the formula Fe2(SR)2(NO)4, where "R" is any alkyl group.[3] In addition Roussin's red salt is discussed in the fields of microbiology and food science due to its mutagenic properties.[8]
The ester derivative are being investigated as nitric oxide donors in biology and medicine, due to the relatively low toxicity and good stability of Roussin's red salt.[9]Photodissociation of the compound induces the release of NO, thereby sensitizing target cells to exposure to radiation.[8]
See also[edit]
References[edit]
- ^ Butler, Anthony R. (July 1982). "The chemist Z. Roussin (1827-94)". Journal of Chemical Education. 59 (7): 549. Bibcode:1982JChEd..59..549B. doi:10.1021/ed059p549.
- ^ Roussin, M. L. (1858). "Recherches sur les nitrosulfures doubles de fer (nouvelle classe de sels)". Ann. Chim. Phys. 52: 285–303.
- ^ a b Thomas, J. T.; Robertson, J. H.; Cox, E. G. (1 September 1958). "The crystal structure of Roussin's red ethyl ester". Acta Crystallographica. 11 (9): 599–604. doi:10.1107/S0365110X58001602.
- ^ Jaworska, Maria; Stasicka, Zofia (2005). "Structure and UV-Vis spectroscopy of the iron-sulfur dinuclear nitrosyl complexes [Fe2S2(NO)4]2− and [Fe2(SR)2(NO)4]". New Journal of Chemistry. 29 (4): 604. doi:10.1039/B409519G.
- ^ Butler, Anthony R. (July 1982). "The chemist Z. Roussin (1827-94)". Journal of Chemical Education. 59 (7): 549. Bibcode:1982JChEd..59..549B. doi:10.1021/ed059p549.
- ^ Hans Reihlen, Adolf v. Friedolsheim (1927). "Über komplexe Stickoxydverbindungen und das sogenannte einwertige Eisen". Justus Liebigs Annalen der Chemie. 457: 71–82. doi:10.1002/jlac.19274570103.
- ^ TB Rauchfuss; TD Weatherill (1982). "Roussin's Red Salt revisited: reactivity of Fe2 (μ-E) 2 (NO) 42-(E= S, Se, Te) and related". Inorganic Chemistry. 21 (2): 827–830. doi:10.1021/ic00132a071.
- ^ a b Greenwood, N. N.; & Earnshaw, A. (1997). Chemistry of the Elements (2nd Edn.), Oxford:Butterworth-Heinemann. ISBN 0-7506-3365-4.
- ^ Yoon, H.; Park, S.; Lim, M. (2020). "Photorelease Dynamics of Nitric Oxide from Cysteine-Bound Roussin's Red Ester". The Journal of Physical Chemistry Letters. 11 (9): 3198–3202. doi:10.1021/acs.jpclett.0c00739. PMID 32250631. S2CID 215408785.