
The Pidgeon process is a practical method for smelting magnesium. The most common method involves the raw material, dolomite being fed into an externally heated reduction tank and then thermally reduced to metallic magnesium using 75% ferrosilicon as a reducing agent in a vacuum.[1] Overall the processes in magnesium smelting via the Pidgeon process involve dolomite calcination, grinding and pelleting, and vacuum thermal reduction.[1] Besides the Pidgeon process, electrolysis of magnesium chloride for commercial production of magnesium is also used, at one point in time accounting for 75% of the world's magnesium production.[2]
Chemistry[edit]
The general reaction that occurs in the Pidgeon process is:
For industrial use, ferrosilicon is used because its cheaper and more readily available than silicon. The iron from the alloy is a spectator in the reaction. CaC2 may also be used as an even cheaper alternative for silicon and ferrosilicon, but is disadvantageous because it decreases the magnesium yield slightly.[3]
The magnesium raw material of this type of reaction is magnesium oxide, which is obtained in many ways. In all cases, the raw materials have to be calcined to remove both water and carbon dioxide. Without doing so, the reaction would be gaseous at reaction temperatures and may even revert the reaction. Magnesium oxide can be obtained by sea or lake water magnesium chloride hydrolyzed to hydroxide. It is calcined to magnesium oxide by removing water. Another option is to use mined magnesite (MgCO3) calcined to magnesium oxide by carbon dioxide removal.
The most used raw material is mined dolomite, a mixed (Ca,Mg)CO3, where the calcium oxide present in the reaction zone scavenges the silica formed, releasing heat and consuming one of the products, ultimately helping push the equilibrium to the right.
(1) Dolomite calcination
(2) Reduction
The Pidgeon process is an endothermic reaction (H° ~183.0kJ/mol Si). Thermodynamically speaking, the temperatures decrease when the vacuum is used for both MgO and calcined dolomite.[3]
Summary of Pidgeon process using dolomite[edit]

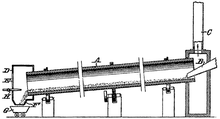
Being an endothermic reaction, heat is applied to initiate and sustain the reaction. This heat requirement may be very high. To keep reaction temperatures low, the processes are operated under pressure. The rotary kiln is typically used in dolomite calcination. In the rotary kiln, the raw material, calcinated dolomite, is mixed with the finely ground reducing agent, ferrosillicone and the catalyst, fluorite. The materials are mixed together and pressed into sphere shaped pellets and the mixed materials are charged into cylindrical nickel chromium steel retorts. A number of retorts are placed in a furnace in sealed paper bags to avoid moisture absorption so that calcined dolomite activity doesn't reduce magnesium yield. The pellets are then placed into a reduction tank and heated to 1200 °C. The inside of the furnace is vacuumed with a 13.3 Pa or higher, to produce magnesium vapour. Magnesium crystals are removed from the condensers, slag is removed as a solid and the retort is recharged. The crude magnesium is refined via flux, and commercial magnesium ingot is produced.[1]
Other routes for magnesium processing[edit]
The carbothermic route to magnesium has been recognized as a low energy, yet high productivity path to magnesium extraction. The chemistry is as follow:
The reaction between magnesia and carbon produces a magnesium and carbon monoxide vapour. A disadvantage of this method is that slow cooling the vapour can cause the reaction to quickly revert back. To prevent this from happening, the magnesium can be dissolved directly in a suitable metal solvent before reversion starts happening. Rapid quenching of the vapour can also be performed to prevent reversion.[4]
The Bolzano process is very similar to the Pidgeon process. The difference between the Pidgeon process and the Bolzano process is that the heating process is done through electric heating conductors, and retorts are placed vertically into large blocks in the Bolzano process.[3] A complete reaction takes about 20–24 hours.[5] The Pidgeon method is less technologically complex and because of distillation/vapour deposition conditions, a high purity product is easily achievable.[3]

Besides the Pigeon process, the second most used process for magnesium production is electrolysis. This is a two step process. The first step is to prepare feedstock containing magnesium chloride and the second step is to dissociate the compound in electrolytic cells as magnesium metal and chlorine gas.[5] The basic reaction is as follows:
The magnesium chloride can be obtained using the Dow process, a process that mixes sea water and dolomite in a flocculator or by dehydration of magnesium chloride brines. The electrolytic cells are partially submerged in a molten salt electrolyte to which the produced magnesium chloride is added in concentrations between 6-18%.[5] The temperatures at which this reaction is operated is between 680 and 750 degrees Celsius.[5] This process does have its fair share of disadvantages including production of harmful chlorine gas and the overall reaction being very energy intensive, creating environmental risks.[6] The Pidgeon process is more advantageous regarding its simplicity, shorter construction period, low power consumption and overall good magnesium quality compared to the electrolysis method.[1]
Disadvantages of the Pidgeon process[edit]
Although the Pidgeon process has many perks, there are some environmental disadvantages of the process as well. Since increased demand for magnesium has risen in recent years, production through ore reduction has been emitting large amounts of carbon dioxide and particulate matter.[7] Due to the lightweight nature of magnesium as well as its high energy density, suggestions have been made about the global consumption of this versatile metal drastically increasing even more than it already has. There are environmental impacts because to create light weight materials in the first place, more energy is needed compared to the material being replaced, typically iron or steel. As an approximate, around 10.4 kg of coal is burned and 37 kg of carbon dioxide is released, per 1 kg of magnesium obtained.[8][9][10] In China, production of magnesium using the Pidgeon process has a 60% higher global warming impact than aluminum, a competing metal mass produced in the country as well.[10] Ultimately, more information and research is needed to make new energy saving changes to reduce the environmental impact of magnesium production on a global scale.
History[edit]
The silicothermic reduction of dolomite was first developed by Amati in 1938 at the University of Padua. Immediately afterward, an industrial production was established in Bolzano (Italy), using what is now better known as the Bolzano process.[11]
A few years later in 1939, when Canada and its allies entered WW2, they were short on supplies that required magnesium such as bombs, other military devices and aluminum alloys needed for aircraft. Dr. Lloyd Montgomery Pidgeon at the National Research Council was able to create a method for extracting magnesium from dolomite in a vacuum at high temperature with ferrosilicon as the reducing agent. At this time, the ferrosilicon method was known, however it had yet to be commercialized. By early 1942, a successful pilot test took place.[12]
Since then, the Pidgeon process has continually been widely used, especially in China, the worlds largest magnesium producer.
References[edit]
- ^ a b c d Wu, Lan’er; Han, Fenglan; Liu, Guiqun (2021), "Magnesium Smelting via the Pidgeon Process", Comprehensive Utilization of Magnesium Slag by Pidgeon Process, SpringerBriefs in Materials, Singapore: Springer Singapore, pp. 45–68, doi:10.1007/978-981-16-2171-0_2, ISBN 978-981-16-2173-4, S2CID 235872413
- ^ Wu, Lan'er (2021). Comprehensive utilization of magnesium slag by pidgeon process. Fenglan Han, Guiqun Liu. Singapore. ISBN 978-981-16-2171-0. OCLC 1249509843.
{{cite book}}: CS1 maint: location missing publisher (link) - ^ a b c d Magnesium and its alloys : technology and applications. Menachem Bamberger, Leszek A. Dobrzański, George E. Totten (First ed.). Boca Raton, FL. 2020. ISBN 978-1-351-04547-6. OCLC 1111577710.
{{cite book}}: CS1 maint: location missing publisher (link) CS1 maint: others (link) - ^ Brooks, Geoffrey; Trang, Simon; Witt, Peter; Khan, M. N. H.; Nagle, Michael (May 2006). "The carbothermic route to magnesium". JOM. 58 (5): 51–55. Bibcode:2006JOM....58e..51B. doi:10.1007/s11837-006-0024-x. ISSN 1047-4838. S2CID 67763716.
- ^ a b c d "Magnesium processing | Techniques & Methods | Britannica". www.britannica.com. Retrieved 2023-04-16.
- ^ Lee, Tae-Hyuk; Okabe, Toru H.; Lee, Jin-Young; Kim, Young Min; Kang, Jungshin (September 2021). "Development of a novel electrolytic process for producing high-purity magnesium metal from magnesium oxide using a liquid tin cathode". Journal of Magnesium and Alloys. 9 (5): 1644–1655. doi:10.1016/j.jma.2021.01.004. S2CID 233930398.
- ^ Wada, Yuji; Fujii, Satoshi; Suzuki, Eiichi; Maitani, Masato M.; Tsubaki, Shuntaro; Chonan, Satoshi; Fukui, Miho; Inazu, Naomi (2017-04-12). "Smelting Magnesium Metal using a Microwave Pidgeon Method". Scientific Reports. 7 (1): 46512. Bibcode:2017NatSR...746512W. doi:10.1038/srep46512. ISSN 2045-2322. PMC 5388895. PMID 28401910.
- ^ Johnson, M. C.; Sullivan, J. L. (2014-09-01). "Lightweight Materials for Automotive Application: An Assessment of Material Production Data for Magnesium and Carbon Fiber": ANL/ESD––14/7, 1172026. doi:10.2172/1172026. OSTI 1172026.
{{cite journal}}: Cite journal requires|journal=(help) - ^ Gao, Feng; Nie, Zuo-ren; Wang, Zhi-hong; Gong, Xian-zheng; Zuo, Tie-yong (June 2008). "Assessing environmental impact of magnesium production using Pidgeon process in China". Transactions of Nonferrous Metals Society of China. 18 (3): 749–754. doi:10.1016/S1003-6326(08)60129-6.
- ^ a b Ramakrishnan, S.; Koltun, P. (August 2004). "Global warming impact of the magnesium produced in China using the Pidgeon process". Resources, Conservation and Recycling. 42 (1): 49–64. doi:10.1016/j.resconrec.2004.02.003. ISSN 0921-3449.
- ^ Magnesium Technology. Berlin/Heidelberg: Springer-Verlag. 2006. doi:10.1007/3-540-30812-1. ISBN 978-3-540-20599-9.
- ^ "Science & Tech Innovations - National Research Council Canada". 2005-02-23. Archived from the original on 2005-02-23. Retrieved 2023-04-16.





