 | |
| Clinical data | |
|---|---|
| Other names | Androstenedione-7α-carboxyethylthioether; 3-[(3,17-Dioxoandrost-4-en-7α-yl)thio]propanoic acid |
| Identifiers | |
| |
| CAS Number | |
| PubChem CID | |
| ChemSpider | |
| Chemical and physical data | |
| Formula | C22H30O4S |
| Molar mass | 390.54 g·mol−1 |
| 3D model (JSmol) | |
| |
| |
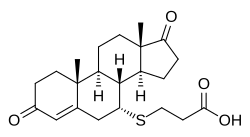
Ovandrotone, also known as androstenedione-7α-carboxyethylthioether,[1] is a synthetic androstane steroid and the C7α carboxyethylthioether of androstenedione.[2] It is a component of ovandrotone albumin (Fecundin), a conjugate of androstenedione and human serum albumin and an immunogen and vaccine against androstenedione that is used to generate androgen immunity and thereby increase the ovulation rate and number of lambs born to ewes.[3][4]
References[edit]
- ^ Journal of Animal Science. American Society of Animal Science. 1991. pp. 3931–3932.
- ^ Elks J (14 November 2014). The Dictionary of Drugs: Chemical Data: Chemical Data, Structures and Bibliographies. Springer. p. 908. ISBN 978-1-4757-2085-3.
- ^ Hoskinson RM, Scaramuzzi RJ, Campbell BK, Downing JA, Welch RJ, Harrison BE (1986). "Effects of Antibodies to Steroid Hormones on Reproductive Events of Sheep and Cattle". Immunological Approaches to Contraception and Promotion of Fertility. Reproductive Biology. Springer. pp. 351–366. doi:10.1007/978-1-4684-5140-5_38. ISBN 978-1-4684-5142-9.
- ^ Carnegie PR (1988). "Autoimmunization Against Hormones: A New Strategy in Animal Production". Anti-Idiotypes, Receptors, and Molecular Mimicry. Springer. pp. 245–254. doi:10.1007/978-1-4612-3734-1_15. ISBN 978-1-4612-8325-6.