Oocyte selection is a procedure that is performed prior to in vitro fertilization, in order to use oocytes with maximal chances of resulting in pregnancy. In contrast, embryo selection takes place after fertilization.
Not all women can conceive naturally, leaving them with a need for technologies and research that can help them have children. Women who might not be able to have their kids naturally may have the option of in vitro fertilization. In vitro fertilization can be a series of treatments that involves the fertilization of a mature egg with a sperm in a laboratory.[1] Oocyte selection is a part the process for in vitro fertilization.[2] An Oocyte is an egg/ovum that is not fully mature or developed and has not been fertilized; Therefore an oocyte is an undeveloped ovum.
Process[edit]
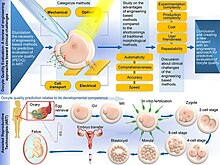
Selecting an oocyte for in vitro fertilization involves assessing the quality of the oocyte which is usually done by accessing the morphological features of the oocyte. The major parts of the oocyte that are accessed for quality in terms of morphological characteristics are the cumulus cells, zona pellucida, polar body, perivitelline space, and cytoplasm; These are the main parts of the oocyte and are usually assessed by conventional microscopy.[3] The size of an oocyte is another factor of the quality of the oocyte; Larger oocyte are usually more quality than smaller ones. [4] Chromosomal evaluation may be performed. Embryos from rescued in vitro-matured metaphase II (IVM-MII) oocytes show significantly higher fertilization rates and more blastomeres per embryo compared with those from arrested metaphase I (MI) oocytes (58.5% vs. 43.9% and 5.7 vs. 5.0, respectively).[5]
Also, morphological features of the oocyte that can be obtained by standard light or polarized light microscopy. However, there is no clear tendency in recent publications to a general increase in predictive value of morphological features.[6] Suggested techniques include zona pellucida imaging, which can detect differences in birefringence between eggs, which is a predictor of compaction, blastulation and pregnancy.[7]
Potentially, polar body biopsy may be used for molecular analysis, and can be used for preimplantation genetic screening.[8]
References[edit]
- ^ "In vitro fertilization (IVF) - Mayo Clinic". www.mayoclinic.org. Retrieved 2024-03-26.
- ^ "Definition of OOCYTE". www.merriam-webster.com. 2024-03-14. Retrieved 2024-03-25.
- ^ Lemseffer Y, Terret ME, Campillo C, Labrune E (September 2022). "Methods for Assessing Oocyte Quality: A Review of Literature". Biomedicines. 10 (9): 2184. doi:10.3390/biomedicines10092184. PMC 9495944. PMID 36140285.
- ^ Wiegel RE, Rubini E, Rousian M, Schoenmakers S, Laven JS, Willemsen SP, et al. (June 2023). "Human oocyte area is associated with preimplantation embryo usage and early embryo development: the Rotterdam Periconception Cohort". Journal of Assisted Reproduction and Genetics. 40 (6): 1495–1506. doi:10.1007/s10815-023-02803-1. PMC 10310608. PMID 37129725.
- ^ Strassburger D, Goldstein A, Friedler S, Raziel A, Kasterstein E, Mashevich M, et al. (August 2010). "The cytogenetic constitution of embryos derived from immature (metaphase I) oocytes obtained after ovarian hyperstimulation". Fertility and Sterility. 94 (3): 971–978. doi:10.1016/j.fertnstert.2009.04.035. PMID 19505687.
- ^ Rienzi L, Vajta G, Ubaldi F (2010). "Predictive value of oocyte morphology in human IVF: a systematic review of the literature". Human Reproduction Update. 17 (1): 34–45. doi:10.1093/humupd/dmq029. PMC 3001337. PMID 20639518.
- ^ Ebner T, Balaban B, Moser M, Shebl O, Urman B, Ata B, et al. (August 2010). "Automatic user-independent zona pellucida imaging at the oocyte stage allows for the prediction of preimplantation development". Fertility and Sterility. 94 (3): 913–920. doi:10.1016/j.fertnstert.2009.03.106. PMID 19439291.
- ^ Jiao ZX, Woodruff TK (June 2013). "Detection and quantification of maternal-effect gene transcripts in mouse second polar bodies: potential markers of embryo developmental competence". Fertility and Sterility. 99 (7): 2055–2061. doi:10.1016/j.fertnstert.2013.02.003. PMC 3672332. PMID 23465709.