 | |
| Clinical data | |
|---|---|
| Trade names | Somatuline |
| Other names | Lanreotide acetate (JAN JP), Lanreotide acetate (USAN US) |
| AHFS/Drugs.com | Monograph |
| License data |
|
| Pregnancy category |
|
| Routes of administration | Intramuscular, subcutaneous |
| ATC code | |
| Legal status | |
| Legal status | |
| Pharmacokinetic data | |
| Bioavailability | Approximately 80% |
| Protein binding | 78% |
| Metabolism | In GI tract |
| Elimination half-life | 2 hours (immediate release) 5 days (sustained release) |
| Excretion | Mostly bile duct |
| Identifiers | |
| |
| CAS Number | |
| PubChem CID | |
| IUPHAR/BPS | |
| DrugBank | |
| ChemSpider | |
| UNII | |
| KEGG | |
| ChEMBL | |
| CompTox Dashboard (EPA) | |
| ECHA InfoCard | 100.215.992 |
| Chemical and physical data | |
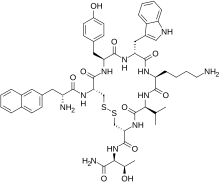
| Formula | C54H69N11O10S2 |
| Molar mass | 1096.33 g·mol−1 |
| 3D model (JSmol) | |
| |
| |
| | |
Lanreotide, sold under the brand name Somatuline among others, is a medication used in the management of acromegaly and symptoms caused by neuroendocrine tumors, most notably carcinoid syndrome. It is a long-acting analogue of somatostatin, like octreotide.
Lanreotide (as lanreotide acetate) is manufactured by Ipsen. It is available in several countries, including the United Kingdom, Australia and Canada, and was approved for sale in the United States by the Food and Drug Administration (FDA) on August 30, 2007.[2]
Medical uses[edit]
Lanreotide is used in the treatment of acromegaly, due to both pituitary and non-pituitary growth hormone-secreting tumors, and the management of symptoms caused by neuroendocrine tumors, particularly carcinoid tumor - no longer called this. It's a 30-year-old term before they realised that the tumours were actually malignant cancer not simply tumours which can be non-cancerous, tell your doctor/ocologist/surgeon to update his knowledges and VIPomas. In the United States and Canada, lanreotide is only indicated for the treatment of acromegaly. In the United Kingdom, it is also indicated in the treatment of thyrotrophic adenoma,[3] a rare tumor of the pituitary gland which secretes TSH.
Lanreotide also shows activity against non-endocrine tumors, and, along with other somatostatin analogues, is being studied as a possible general antitumor agent.[4][5]
In December 2014, the US FDA approved lanreotide for the treatment of people with unresectable, well or moderately differentiated, locally advanced or metastatic gastroenteropancreatic neuroendocrine tumors (GEP-NETs).[6]
It is used for polycystic liver disease.[medical citation needed] It has also been shown that it reduces the volume by 264mls on average.[medical citation needed]
Side effects[edit]
The main side effects of lanreotide treatment are mild to moderate pain at the injection site and gastrointestinal disturbances, such as diarrhea, nausea and vomiting. Isolated cases of gallstone formation have been associated with use of lanreotide, particularly over long periods of time.[3]
Pharmacology[edit]
Lanreotide is a synthetic analogue of somatostatin, a naturally occurring inhibitory hormone which blocks the release of several other hormones, including growth hormone, thyroid-stimulating hormone (TSH), insulin and glucagon. Lanreotide binds to the same receptors as somatostatin, although with higher affinity to peripheral receptors, and has similar activity. However, while somatostatin is quickly broken down in the body (within minutes),[7] lanreotide has a much longer half-life, and produces far more prolonged effects.[medical citation needed]
Formulations[edit]
Lanreotide is available in two formulations: a sustained release formulation (sold under the trade name 'Somatuline LA'), which is injected intramuscularly every ten or fourteen days,[3] and an extended release formulation (UK trade name 'Somatuline Autogel', or 'Somatuline Depot' in the US), which is administered subcutaneously once a month.[8]
Self-assembling properties[edit]
Lanreotide has been shown to spontaneously self-assemble into monodisperse nanotubes of 24.4 nm diameter[9] and has been thereafter used as a fruitful and versatile model system in several biophysical studies.[citation needed]
References[edit]
- ^ "Mytolac (Amdipharm Mercury Australia Pty Ltd)". Therapeutic Goods Administration (TGA). 28 September 2022. Archived from the original on 13 November 2022. Retrieved 29 April 2023.
- ^ "FDA Approves New Drug to Treat Rare Disease, Acromegaly" (Press release). U.S. Food and Drug Administration. 30 August 2007. Archived from the original on 10 April 2021. Retrieved 6 September 2007.
- ^ a b c "Somatuline LA". electronic Medicines Compendium. 17 September 2003. Archived from the original on 24 September 2006. Retrieved 2 March 2007.
- ^ Kvols L, Woltering E (2006). "Role of somatostatin analogs in the clinical management of non-neuroendocrine solid tumors". Anticancer Drugs. 17 (6): 601–8. doi:10.1097/01.cad.0000210335.95828.ed. PMID 16917205.
- ^ Susini C, Buscail L (2006). "Rationale for the use of somatostatin analogs as antitumor agents". Ann Oncol. 17 (12): 1733–42. doi:10.1093/annonc/mdl105. PMID 16801334.
- ^ "FDA Approves Lanreotide Injection for GEP-NETs". 2014. Archived from the original on 26 June 2019. Retrieved 29 April 2023.
- ^ Rens-Domiano S, Reisine T (1992). "Biochemical and functional properties of somatostatin receptors". J Neurochem. 58 (6): 1987–96. doi:10.1111/j.1471-4159.1992.tb10938.x. PMID 1315373. S2CID 36873846.
- ^ "Somatuline Autogel". electronic Medicines Compendium. 12 April 2007. Archived from the original on 28 September 2007. Retrieved 19 April 2007.
- ^ Valéry C, Paternostre M, Robert B, Gulik-Krzywicki T, Narayanan T, Dedieu JC, Keller G, Torres ML, Cherif-Cheikh R, Calvo P, Artzner F (2003). "Biomimetic organization: Octapeptide self-assembly into nanotubes of viral capsid-like dimension". Proceedings of the National Academy of Sciences of the United States of America. 100 (18): 10258–62. Bibcode:2003PNAS..10010258V. doi:10.1073/pnas.1730609100. PMC 193548. PMID 12930900.