| |

| |
| Names | |
|---|---|
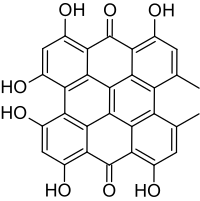
| Preferred IUPAC name
1,3,4,6,8,13-Hexahydroxy-10,11-dimethylphenanthro[1,10,9,8-opqra]perylene-7,14-dione | |
| Other names
4,5,7,4',5',7'-Hexahydroxy-2,2'-dimethylnaphthodianthrone
| |
| Identifiers | |
3D model (JSmol)
|
|
| ChEBI | |
| ChEMBL | |
| ChemSpider | |
| ECHA InfoCard | 100.008.129 |
| KEGG | |
PubChem CID
|
|
| UNII | |
CompTox Dashboard (EPA)
|
|
| |
| |
| Properties | |
| C30H16O8 | |
| Molar mass | 504.450 g·mol−1 |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa).
| |
Hypericin is a naphthodianthrone, an anthraquinone derivative which, together with hyperforin, is one of the principal active constituents of Hypericum (Saint John's wort).[2][3] Hypericin is believed to act as an antibiotic, antiviral[2] and non-specific kinase inhibitor. Hypericin may inhibit the action of the enzyme dopamine β-hydroxylase, leading to increased dopamine levels,[citation needed] although thus possibly decreasing norepinephrine and epinephrine.
It was initially believed[according to whom?] that the anti-depressant pharmacological activity of hypericin was due to inhibition of monoamine oxidase enzyme. The crude extract of Hypericum is a weak inhibitor of MAO-A and MAO-B.[4][5] Isolated hypericin does not display this activity, but does have some affinity for NMDA receptors.[citation needed] This points in the direction that other constituents are responsible for the MAOI effect. The current belief is that the mechanism of antidepressant activity is due to the inhibition of re-uptake of certain neurotransmitters.[2]
The large chromophore system in the molecule means that it can cause photosensitivity when ingested beyond threshold amounts.[citation needed] Photosensitivity is often seen in animals that have been allowed to graze on St. John's Wort.[citation needed] Because hypericin accumulates preferentially in cancerous tissues, it is also used as an indicator of cancerous cells.[citation needed] In addition, hypericin is under research as an agent in photodynamic therapy, whereby a biochemical is absorbed by an organism to be later activated with spectrum-specific light from specialized lamps or laser sources, for therapeutic purposes.[citation needed] The antibacterial and antiviral effects of hypericin are also believed to arise from its ability for photo-oxidation of cells and viral particles.[2]
Hypericin derives from cyclisation of polyketides.[6][7]
The biosynthesis of hypericins is through the polyketide pathway where an octaketide chain goes through successive cyclizations and decarboxylations to form emodin anthrone which is believed to be the precursor of hypericin. Oxidization reactions yield protoforms which then are converted into hypericin and pseudohypericin. These reactions are photosensitive and take place under exposure to light and using the enzyme Hyp-1.[8][9][10][11][12]
References[edit]
- ^ Merck Index, 11th Edition, 4799
- ^ a b c d Mehta S (2012-12-18). "Pharmacognosy of St. John's Wort". Pharmaxchange.info. Retrieved 2014-02-16.
- ^ Oubre A (1991). "Hypericin: the active ingredient in Saint John's Wort". Archived from the original on September 28, 2007. Retrieved September 18, 2006.
- ^ Thiede HM, Walper A (October 1994). "Inhibition of MAO and COMT by hypericum extracts and hypericin". Journal of Geriatric Psychiatry and Neurology. 7 (Suppl 1): S54–56. doi:10.1177/089198879400700114. ISSN 0891-9887. PMID 7857510. S2CID 208042437.
- ^ Bladt S, Wagner H (October 1994). "Inhibition of MAO by fractions and constituents of hypericum extract". Journal of Geriatric Psychiatry and Neurology. 7 (Suppl 1): S57–59. doi:10.1177/089198879400700115. ISSN 0891-9887. PMID 7857511. S2CID 23531061.
- ^ Loren W. Walker (1999). "A Review of the Hypothetical Biogenesis and Regulation of Hypericin synthesis via the Polyketide Pathway in Hypericum perforatum and Experimental Methods Proposed to Evaluate the Hypothesis". Archived from the original on 2019-06-26. Retrieved 2011-01-04.
{{cite journal}}: Cite journal requires|journal=(help) - ^ Christian Hertweck (2009). "Polyketide Biosynthesis". Angew. Chem. Int. Ed. 48 (26): 4688–4716. doi:10.1002/anie.200806121. PMID 19514004.
- ^ Karioti A, Bilia AR (2010). "Hypericins as potential leads for new therapeutics". Int J Mol Sci. 11 (2): 562–594. doi:10.3390/ijms11020562. PMC 2852855. PMID 20386655.
- ^ Falk H (1999). "From the Photosensitizer Hypericin to the Photoreceptor Stentorin- The Chemistry of Phenanthroperylene Quinones". Angew. Chem. Int. Ed. Engl. 38 (21): 3116–3136. doi:10.1002/(SICI)1521-3773(19991102)38:21<3116::AID-ANIE3116>3.0.CO;2-S. PMID 10556884.
- ^ Bais HP, Vepachedu R, Lawrence CB, Stermitz FR, Vivanco JM (2003). "Molecular and biochemical characterization of an enzyme responsible for the formation of hypericin in St. John's wort (Hypericum perforatum L.)". J. Biol. Chem. 278 (34): 32413–32422. doi:10.1074/jbc.M301681200. PMID 12799379.
- ^ Michalska K, Fernandes H, Sikorski M, Jaskolski M (2010). "Crystal structure of Hyp-1, a St. John's wort protein implicated in the biosynthesis of hypericin". J. Struct. Biol. 169 (2): 161–171. doi:10.1016/j.jsb.2009.10.008. PMID 19853038.
- ^ Murthy HN, Kim YS, Park SY, Paek KY (2014). "Hypericins: biotechnological production from cell and organ cultures". Appl. Microbiol. Biotechnol. 98 (22): 9187–9198. doi:10.1007/s00253-014-6119-3. PMID 25301586. S2CID 17487401.