Size of this preview: 755 × 599 pixels. Other resolutions: 302 × 240 pixels | 605 × 480 pixels | 968 × 768 pixels | 1,280 × 1,016 pixels | 1,552 × 1,232 pixels.
Original file (1,552 × 1,232 pixels, file size: 143 KB, MIME type: image/png)
File history
Click on a date/time to view the file as it appeared at that time.
| Date/Time | Thumbnail | Dimensions | User | Comment | |
|---|---|---|---|---|---|
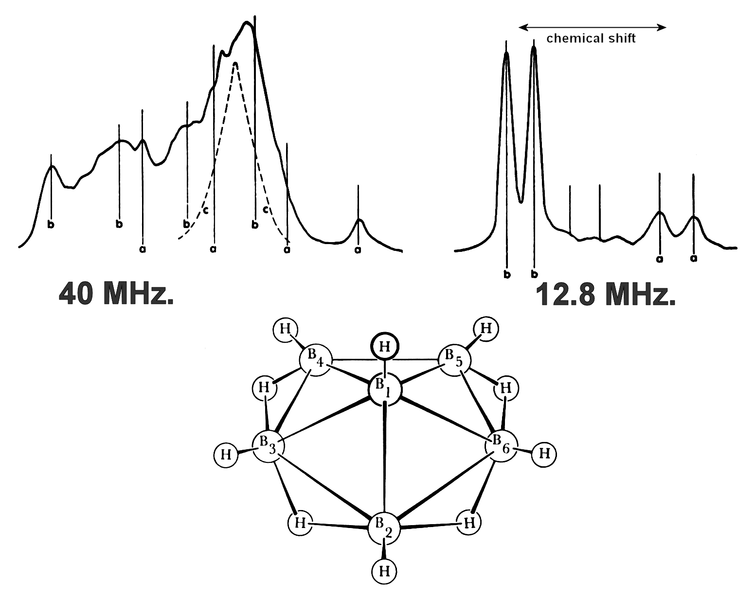
| current | 16:46, 8 March 2011 |  | 1,552 × 1,232 (143 KB) | Jslipscomb | Arrows show the chemical shift |
| 23:57, 5 March 2011 |  | 1,552 × 1,232 (140 KB) | Jslipscomb | Sharper, less blurry. | |
| 01:36, 18 February 2011 |  | 1,552 × 1,232 (184 KB) | Jslipscomb | {{Information |Description ={{en|1=Nuclear Magnetic Resonance NMR spectrum and structure of hexaborane B6H10. Research paid for by a U.S. Government grant.}} |Source =William Lipscomb |Author =WIlliam Lipscomb and Gareth Eaton, edited b |
File usage
The following pages on the English Wikipedia use this file (pages on other projects are not listed):
Global file usage
The following other wikis use this file:
- Usage on ja.wikipedia.org
- Usage on ru.wikipedia.org
- Usage on sr.wikipedia.org
- Usage on tr.wikipedia.org
- Usage on zh.wikipedia.org
