| |
| Names | |
|---|---|
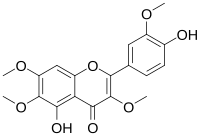
| IUPAC name
4′,5-Dihydroxy-3,3′,6,7-tetramethoxyflavone
| |
| Systematic IUPAC name
5-Hydroxy-4-(4-hydroxy-3-methoxyphenyl)-3,6,7-trimethoxy-4H-1-benzopyran-4-one | |
| Other names
Chrysosplenetin B
3,6,7,3'-Tetra-methylquercetagetin Quercetagetin 3,6,7,3'-tetramethyl ether | |
| Identifiers | |
3D model (JSmol)
|
|
| ChemSpider | |
PubChem CID
|
|
| UNII | |
CompTox Dashboard (EPA)
|
|
| |
| |
| Properties | |
| C19H18O8 | |
| Molar mass | 374.345 g·mol−1 |
| Density | 1.448 g/mL |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa).
| |
Chrysosplenetin is an O-methylated flavonol. It can be found in the root of Berneuxia thibetica and in Chamomilla recutita.[1]
References[edit]
- ^ Miroslav Repčák, Vanda Švehlı́ková, Ján Imrich, Kalevi Pihlaja (1999). "Jaceidin and chrysosplenetin chemotypes of Chamomilla recutita (L.) Rauschert". Biochemical Systematics and Ecology. 27 (7): 727–732. doi:10.1016/S0305-1978(98)00124-0.
{{cite journal}}: CS1 maint: multiple names: authors list (link)