| |
| |
| Names | |
|---|---|
| Preferred IUPAC name
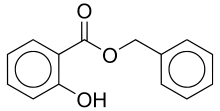
Benzyl 2-hydroxybenzoate | |
| Identifiers | |
3D model (JSmol)
|
|
| ChEMBL | |
| ChemSpider | |
| ECHA InfoCard | 100.003.876 |
PubChem CID
|
|
| UNII | |
CompTox Dashboard (EPA)
|
|
| |
| |
| Properties | |
| C14H12O3 | |
| Molar mass | 228.247 g·mol−1 |
| Appearance | Colorless liquid |
| Density | 1.17 g/cm3 |
| Melting point | 24 to 25 °C (75 to 77 °F; 297 to 298 K) |
| Boiling point | 318 °C (604 °F; 591 K) |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa).
| |
Benzyl salicylate is a salicylic acid benzyl ester, a chemical compound most frequently used in cosmetics as a fragrance additive or UV light absorber. It appears as an almost colorless liquid with a mild odor described as "very faint, sweet-floral, slightly balsamic" by some, while others smell nothing at all. There is debate whether the odour is caused solely by impurities or a genetic predisposition.[1] It occurs naturally in a variety of plants and plant extracts and is widely used in blends of fragrance materials.[2]
There is some evidence that people may become sensitized to this material[3] and as a result, there is a restriction standard concerning the use of this material in fragrances by the International Fragrance Association.[4]
It is used as a solvent for crystalline synthetic musks and as a component and fixative in floral perfumes such as carnation, jasmine, lilac, and wallflower.[5]
See also[edit]
References[edit]
- ^ Steffen Arctander: Perfume and Flavor Chemicals. ISBN 0-931710-37-5
- ^ "Benzyl salicylate". The Good Scents Company.
- ^ Belsito, D; Bickers, D; Bruze, M; Calow, P; Greim, H; Hanifin, JM; Rogers, AE; Saurat, JH; Sipes, IG; Tagami, H (2007). "Toxicologic and Dermatologic Assessments for Three Groups of Fragrance Ingredients: 1) Related Esters and Alcohols of Cinnamic Acid and Cinnamic Alcohol, 2) Ionones, 3) Salicylates" (PDF). Food and Chemical Toxicology. 45 (Supplement 1): S1-23. doi:10.1016/j.fct.2007.09.087. PMID 18035463. Archived from the original (PDF) on 2021-01-12. Retrieved 2012-04-05.
- ^ "Standards Restricted". International Fragrance Association. Archived from the original on 2012-01-04.
- ^ An Introduction to Perfumery by Curtis & Williams 2nd Edition, 2009, ISBN 978-0-9608752-8-3, ISBN 978-1-870228-24-4