| |
| Names | |
|---|---|
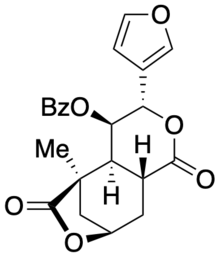
| Systematic IUPAC name
(3S,4R,4aS,5R,8R,9aR)-4-(benzoyloxy)-3-(3-furanyl)hexahydro-5-methyl-5,8-Methano-1H-pyrano[3,4-d]oxepin-1,6(5H)-dione | |
Other names
| |
| Identifiers | |
3D model (JSmol)
|
|
| ChemSpider | |
PubChem CID
|
|
| |
| |
| Properties | |
| C22H20O7 | |
| Molar mass | 396.395 g·mol−1 |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa).
| |
Collybolide is a secondary metabolite of the Rhodocollybia maculata mushroom, a basidiomycete fungus that grows on rotting conifer wood. It was previously believed to be a potent and selective kappa-opioid receptor agonist.[1] However, a total synthesis and independent biological assay determined that collybolide neither excites nor suppresses kappa-opioid receptor signaling.[2] Collybolide is unlikely to be psychoactive, although it has been shown to inhibit L-type calcium channels in isolated rat aorta.[3]
History[edit]
Collybolide was first isolated from R. maculata in 1911,[4][5][6] but its structure remained unsolved until the 1970s, when X-ray crystallography yielded the structure of a collybolide epimer, isocollybolide,[7] and 1H and 13C NMR elucidated the structure and relative stereochemistry of collybolide.[8] Importantly, early reports were unable to confidently assign the absolute configuration of collybolide; a 1986 isolation of a collybolide congener noted that the absolute configuration of the series "remains to be determined",[9] and a 2001 circular dichroism study was only able to tentatively infer which enantiomer naturally occurred based on density functional theory calculations.[10] A 2016 report claimed to have conclusively assigned the absolute configuration of collybolide by X-ray crystallography,[1] but a following 2022 report noted that the Flack parameter accompanying the 2016 crystal structure was inconclusive,[2] and could not be used to confidently assign its absolute stereochemistry.
Purported kappa-opioid receptor agonism[edit]
Collybolide is a sesquiterpene that contains a furyl-δ-lactone, a structural feature shared with the diterpene natural product salvinorin A. Salvinorin A is a hallucinogen that acts via high-potency agonism of the human kappa-opioid receptor (KOR), and collybolide's structural similarity to salvinorin A prompted a 2016 team to investigate collybolide's activity at the KOR, in the hopes of discovering a new, non-nitrogenous opioid.[1] Radioligand displacement and functional assays showed collybolide binding to (Ki = 0.9 nM) and activating the human KOR, and an in vivo assay described collybolide inhibiting chloroquine-induced itch in mice at an extremely low dose (IC50 = 0.08 mg/kg). These results attracted widespread attention in the biomedical community, as collybolide appeared to be a potent and selective KOR agonist that might be developed into a new treatment for pain or pruritus,[11][12] lacking the adverse effects of typical mu-opioid receptor agonist pain treatments. These claims of KOR agonism also attracted the attention of the recreational psychedelic community.[13]
Independent chemical synthesis and biological assay of collybolide in 2022 found that it was devoid of opioid activity.[2] Radioligand displacement assays showed only weak (Ki = 794 nM) binding of collybolide to the human KOR, and functional assays showed that collybolide does not activate KOR signaling at concentrations up to 10 μM (measured by [35S]GTPγS binding, cAMP accumulation, and beta-arrestin recruitment assays). Shevick et al. note the presence of surface-modifying agents in the 2016 assay procedures, in addition to low percent stimulation in the 2016 [35S]GTPγS assay, that may have caused noise in the data to be mistaken as signal.[2] The source of the false positive result for KOR agonism in the 2016 study has yet to be rigorously identified. However, the findings and conclusions of the 2022 study – that collybolide was incorrectly assigned as a KOR agonist – explain why no credible reports of collybolide's psychoactivity have surfaced.[14][15]
Chemical synthesis[edit]
The 2022 reevaluation of collybolide's KOR activity leveraged access to both natural and unnatural enantiomers of collybolide via total synthesis.[2][16] Key features of the synthesis included an enantioselective Diels-Alder reaction using the Hayashi-Jørgensen proline organocatalyst, and an enamine [3,3]-sigmatropic rearrangement to stereoselectively install a late-stage benzoyloxy (BzO) group.

References[edit]
- ^ a b c Gupta, Achla; Gomes, Ivone; Bobeck, Erin N.; Fakira, Amanda K.; Massaro, Nicholas P.; Sharma, Indrajeet; Cavé, Adrien; Hamm, Heidi E.; Parello, Joseph; Devi, Lakshmi A. (9 May 2016). "Collybolide is a novel biased agonist of κ-opioid receptors with potent antipruritic activity". Proceedings of the National Academy of Sciences. 113 (21): 6041–6046. Bibcode:2016PNAS..113.6041G. doi:10.1073/pnas.1521825113. PMC 4889365. PMID 27162327.
- ^ a b c d e Shevick, Sophia L.; Freeman, Stephan M.; Tong, Guanghu; Russo, Robin J.; Bohn, Laura M.; Shenvi, Ryan A. (27 July 2022). "Asymmetric Syntheses of (+)- and (−)-Collybolide Enable Reevaluation of kappa -Opioid Receptor Agonism". ACS Central Science. 8 (7): 948–954. doi:10.1021/acscentsci.2c00442. PMC 9335922. PMID 35912357.
- ^ Hansske, Friedrich; Paululat, Thomas; Gerlitz, Martin; Gruen-Wollny, Iris (June 9, 2005). "WO2005051376 - Arzneimittel enthaltend Collybolide". WIPO. Archived from the original on May 11, 2023. Retrieved May 11, 2023.
{{cite web}}: CS1 maint: bot: original URL status unknown (link) - ^ Goris, A.; Mascre, M. (1911). "Sur la composition chimique de quelques Champignons superieurs". Comptes rendus hebdomadaires des séances de l'Académie des sciences. 153: 1082–1084.
- ^ Pascard-Billy, Claudine (1970). "X-Ray crystallographic determination of the structure of the sesquiterpenoid isocollybolide". Journal of the Chemical Society D: Chemical Communications (24): 1722a. doi:10.1039/c2970001722a. ISSN 0577-6171.
- ^ Ayer, William A.; Browne, Lois M. (1981-01-01). "Terpenoid metabolites of mushrooms and related basidiomycetes". Tetrahedron. 37 (12): 2197–2248. doi:10.1016/S0040-4020(01)97979-7. ISSN 0040-4020.
- ^ Pascard-Billy, Claudine (1970). "X-Ray crystallographic determination of the structure of the sesquiterpenoid isocollybolide". Journal of the Chemical Society D: Chemical Communications (24): 1722a. doi:10.1039/c2970001722a. ISSN 0577-6171.
- ^ Bui, A. -M.; Cavé, A.; Janot, M. -M.; Parello, J.; Potier, P.; Scheidegger, U. (1974-01-01). "Isolement et analyse structurale du collybolide, nouveau sesquiterpene extrait de Collybia maculata alb. et sch. ex fries (basidiomycetes)". Tetrahedron (in French). 30 (11): 1327–1336. doi:10.1016/S0040-4020(01)97243-6. ISSN 0040-4020.
- ^ Fogedal, Mats; Norberg, Thomas (1986-01-01). "Deoxycollybolidol, a sesquiterpene from collybia peronata". Phytochemistry. 25 (11): 2661–2663. doi:10.1016/S0031-9422(00)84533-1. ISSN 0031-9422.
- ^ Castronovo, Francesca; Clericuzio, Marco; Toma, Lucio; Vidari, Giovanni (2001-04-02). "Fungal metabolites. Part 45: The sesquiterpenes of Collybia maculata and Collybia peronata". Tetrahedron. 57 (14): 2791–2798. doi:10.1016/S0040-4020(01)00120-X. ISSN 0040-4020.
- ^ Santino, Federica; Gentilucci, Luca (January 2023). "Design of κ-Opioid Receptor Agonists for the Development of Potential Treatments of Pain with Reduced Side Effects". Molecules. 28 (1): 346. doi:10.3390/molecules28010346. ISSN 1420-3049. PMC 9822356. PMID 36615540.
- ^ Chakraborty, Soumen; Majumdar, Susruta (2021-05-11). "Natural Products for the Treatment of Pain: Chemistry and Pharmacology of Salvinorin A, Mitragynine, and Collybolide". Biochemistry. 60 (18): 1381–1400. doi:10.1021/acs.biochem.0c00629. ISSN 0006-2960. PMC 7982354. PMID 32930582.
- ^ "Anyone know about collybia maculata?". Reddit. October 5, 2020. Archived from the original on May 11, 2023. Retrieved May 10, 2023.
{{cite web}}: CS1 maint: bot: original URL status unknown (link) - ^ Khan, Md Imdadul H.; Sawyer, Benjamin J.; Akins, Nicholas S.; Le, Hoang V. (2022-12-05). "A systematic review on the kappa opioid receptor and its ligands: New directions for the treatment of pain, anxiety, depression, and drug abuse". European Journal of Medicinal Chemistry. 243: 114785. doi:10.1016/j.ejmech.2022.114785. ISSN 0223-5234. PMID 36179400. S2CID 252476850.
- ^ Santino, Federica; Gentilucci, Luca (January 1, 2023). "Design of κ-Opioid Receptor Agonists for the Development of Potential Treatments of Pain with Reduced Side Effects". Molecules. 28 (1): 346. doi:10.3390/molecules28010346. ISSN 1420-3049. PMC 9822356. PMID 36615540.
- ^ "Synthesis and Reevaluation of (+)- and (−)-Collybolide as kappa-Opioid Receptor Agonists". Synfacts. 18 (11): 1251. October 18, 2022. doi:10.1055/s-0041-1738630. ISSN 1861-1958. S2CID 253010900.