 | |
| Identifiers | |
|---|---|
| |
| CAS Number | |
| PubChem CID | |
| ChemSpider | |
| UNII | |
| ChEMBL | |
| CompTox Dashboard (EPA) | |
| Chemical and physical data | |
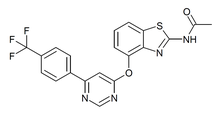
| Formula | C20H13F3N4O2S |
| Molar mass | 430.41 g·mol−1 |
| 3D model (JSmol) | |
| |
| |
AMG-517 is a drug which acts as a potent and selective blocker of the TRPV1 ion channel.[1][2] It was developed as a potential treatment for chronic pain, but while it was an effective analgesic in animal studies it was dropped from human clinical trials at Phase I due to producing hyperthermia as a side effect, as well as poor water solubility. It is still used in scientific research into the function of the TRPV1 channel and its role in pain and inflammation, and has been used as a template for the design of several newer analogues which have improved properties.[3][4][5][6][7]
See also[edit]
References[edit]
- ^ Doherty EM, Fotsch C, Bannon AW, Bo Y, Chen N, Dominguez C, et al. (July 2007). "Novel vanilloid receptor-1 antagonists: 2. Structure-activity relationships of 4-oxopyrimidines leading to the selection of a clinical candidate". Journal of Medicinal Chemistry. 50 (15): 3515–27. doi:10.1021/jm070190p. PMID 17585750.
- ^ Wang HL, Katon J, Balan C, Bannon AW, Bernard C, Doherty EM, et al. (July 2007). "Novel vanilloid receptor-1 antagonists: 3. The identification of a second-generation clinical candidate with improved physicochemical and pharmacokinetic properties". Journal of Medicinal Chemistry. 50 (15): 3528–39. doi:10.1021/jm070191h. PMID 17585751.
- ^ Wang X, Chakrabarti PP, Ognyanov VI, Pettus LH, Tamir R, Tan H, et al. (December 2007). "Trisubstituted pyrimidines as transient receptor potential vanilloid 1 (TRPV1) antagonists with improved solubility". Bioorganic & Medicinal Chemistry Letters. 17 (23): 6539–45. doi:10.1016/j.bmcl.2007.09.080. PMID 17937985.
- ^ Gavva NR, Treanor JJ, Garami A, Fang L, Surapaneni S, Akrami A, Alvarez F, Bak A, Darling M, Gore A, Jang GR, Kesslak JP, Ni L, Norman MH, Palluconi G, Rose MJ, Salfi M, Tan E, Romanovsky AA, Banfield C, Davar G (May 2008). "Pharmacological blockade of the vanilloid receptor TRPV1 elicits marked hyperthermia in humans". Pain. 136 (1–2): 202–10. doi:10.1016/j.pain.2008.01.024. PMID 18337008. S2CID 11557845.
- ^ Gavva NR (November 2008). "Body-temperature maintenance as the predominant function of the vanilloid receptor TRPV1". Trends in Pharmacological Sciences. 29 (11): 550–7. doi:10.1016/j.tips.2008.08.003. PMID 18805596.
- ^ Garami A, Ibrahim M, Gilbraith K, Khanna R, Pakai E, Miko A, et al. (November 2017). "Transient Receptor Potential Vanilloid 1 Antagonists Prevent Anesthesia-induced Hypothermia and Decrease Postincisional Opioid Dose Requirements in Rodents". Anesthesiology. 127 (5): 813–823. doi:10.1097/ALN.0000000000001812. PMID 28806222. S2CID 38802079.
- ^ Tsagareli MG, Nozadze I, Tsiklauri N, Carstens MI, Gurtskaia G, Carstens E (November 2020). "Thermal Hyperalgesia and Mechanical Allodynia Elicited by Histamine and Non-histaminergic Itch Mediators: Respective Involvement of TRPV1 and TRPA1". Neuroscience. 449: 35–45. doi:10.1016/j.neuroscience.2020.09.048. PMC 8219216. PMID 33010342.